2019 Volume 2 Issue 2
|
|
INEOS OPEN, 2019, 2 (2), 41–44 Journal of Nesmeyanov Institute of Organoelement Compounds DOI: 10.32931/io1907a |
|
Synthesis of Biologically Relevant Complexes Based on
Fullerene and Vitamin B12 Derivative
D. V. Beigulenko,b I. A. Yamskov,a and K. A. Kochetkov*a,b
a Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, Moscow, 119991 Russia
b Mendeleev University of Chemical Technology of Russia, Miusskaya pl. 9, Moscow, 125047 Russia
Corresponding author: V. S. Romanova, e-mail: roman@ineos.ac.ru; K. A. Kochetkov, e-mail: const@ineos.ac.ru
Received 2 July 2018; accepted 27 December 2018
Abstract
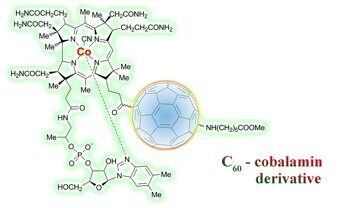
Functionalization of a macrocyclic cobalt complex based on cobalamin at the carboxyl group with an amino acid derivative of fullerene gives rise to a new type of mixed biologically relevant cobalamin–fullerene complexes. The structures of the resulting compounds are elucidated by UV-vis, IR and CD spectroscopies.
Key words: amino acid derivatives of fullerene, cobalamin.
Introduction
For a number of years, our group research has been focused on the synthesis of biologically active compounds based on transition metal complexes that are able to catalyze the processes which involve biological substrates under conditions close to physiological ones. Transition metal complexes are known to serve as efficient catalysts for a range of chemical reactions that resemble the processes in living organisms which occur under action of metalloenzymes [1].
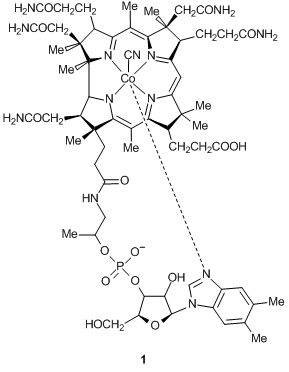
According to the concept suggested by M. E. Vol'pin [2], catalytic sources of reactive oxygen species (ROS), which tend to accumulate selectively in tumors, can significantly inhibit the growth of cancer cells. Of particular interest are natural macrocyclic cobalt complexes, for example, vitamin В12 and its derivatives (cobalamins, in particular, vitamin B12 е-carboxylic acid: e-COOH-Cbl-CN 1). Our recent investigations on the catalytic activity of related complexes in oxidation of natural substrates [3], formation of ROS [4], and oxidative cleavage of nucleic acids [5–7] as well as medical and biological assays on anticancer activity of corrin cobalt complexes and their compositions with l-ascorbic acid confirmed that the catalytic systems based on cobalt complexes with corrin ligands and l-ascorbic acid, which generate reactive oxygen species, can be used as efficient anticancer and related agents [8].
Mono(amino acid) and peptide derivatives of fullerene, synthesized for the first time at INEOS RAS [9], appeared to be nontoxic and exhibited a broad spectrum of biological activity. The most promising compounds with anticancer and antimetastatic properties were found to be the compounds that include nitro groups or carnosine residues [10, 11].
We assumed that the introduction of an active form of vitamin В12 into a molecule of a pharmacophoric fullerene derivative would essentially improve the biological activity of the resulting complexes. For this purpose, it is important to show the principal possibility of synthesis of these compounds.
Results and discussion
The first step of our investigations was concerned with e-COOH-Cbl-CN 1 and N-(monohydrofullerenyl)-ε-aminocaproic acid methyl ester HC60NH(CH2)5COOMe 2.
It is known that fullerene derivatives have an active hydrogen atom in a С60 core which can be readily substituted for the halogen atoms [12–14]. Therefore, for direct attachment of e-COOH-Cbl-CN 1 to a fullerene core, we firstly converted it to the corresponding chloride upon refluxing with a 1.5-fold excess of thionyl chloride in dimethylformamide for 2 h (Scheme 1). The excess of thionyl chloride was removed under vacuum, and the resulting solution was added to a 1.5-fold excess of compound 2 in pyridine. The reaction mixture was stirred at room temperature for 8 h and dialyzed against water. Product 3, CN-Cbl-C(O)-C60NH(CH2)5COOMe, appeared to be soluble in water, therefore, the excess of starting ester 2, which does not dissolve in water, can be readily removed by filtration, whereas unreacted compound 1 moves into a dialysis bag.
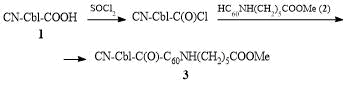
Scheme 1. Synthesis of cobalamin–fullerene complex 3.
The second derivative was obtained by the attachment of 1 to 2 after introduction of a spacer, an ethylene unit, which afforded compound 4 with the following formula: CN-Cbl-C(O)OCH2CH2-C60NH(CH2)5COOMe (Scheme 2). For this purpose, the carboxyl group of compound 1 was firstly modified with 2-chloroethanol. The reaction was carried out in the presence of N,N'-dicyclohexylcarbodiimide (DCC) under stirring at room temperature for 8 h. The resulting solution was added to a solution of a 1.5-fold excess of compound 2 in pyridine. The reaction mixture was stirred at room temperature for 8 h and dialyzed to give compound 4. The latter also appeared to be soluble in water; therefore, its purification was carried out analogously to the above-described procedure.
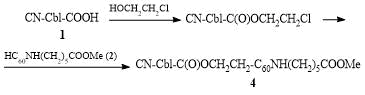
Scheme 2. Synthesis of cobalamin–fullerene complex 4.
The structures of the compounds obtained were elucidated using IR and UV-vis spectroscopies as well as circular dichroism (CD).
The comparison of the CD spectrum of compound 1 with those of fullerene derivatives 3 and 4 revealed some changes (Figs. 1, 2). Thus, the spectrum of compound 3 display, along with the extreme points typical for starting compound e-COOH-Cbl-CN 1, new bands at 471 and 542 nm, which are characteristic for p–p* electronic transitions of fullerene derivatives [15, 16]. Furthermore, the Cotton effect (CE) changes the sign to a negative one in the short-wave region. The negative value of CE was also observed in the CD spectrum of compound 4 at the wavelength below 400 and above 470 nm. The extreme points at 471 and 542 nm, attributed to p–p* electronic transitions of fullerene derivatives, were also detected in the CD spectrum of 4, but were less expressed than in the spectrum of compound 3. This is likely to be connected with a very strong conjugation between the cobalamin moiety and the fullerene core in compound 3 unlike product 4, where the cobalt complex is attached through the ethylene bridge. At the same time, the band at 430 nm, which refers to vitamin B12 е-carboxylic acid, is well expressed in the spectra of all three compounds. The above-mentioned data confirm the attachment of the vitamin В12 derivative to the fullerene core.
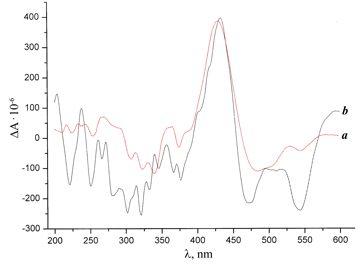
Figure 1. CD spectra of compounds 1 (a) and 3 (b).
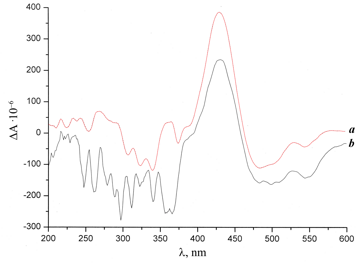
Figure 2. CD spectra of compounds 1 (a) and 4 (b).
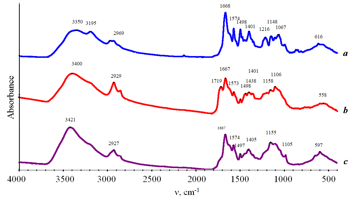
Figure 3. IR spectra of compounds 1 (a), 3 (b) and 4 (c).
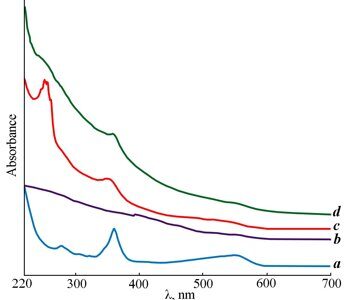
Figure 4. UV-vis spectra of compounds 1 (a), 2 (b), 3 (c) and 4 (d).
Table 1. Characteristic absorption bands in the IR spectra of compounds 1, 3 and 4 (cm–1)
|
Compound |
ν(O–H), ν(N–H) |
ν(C=О) in Cbl |
Absorption bands of amide groups in Cbl |
ν(C≡N) in CN-Cbl |
ν(C–O) in (СООН)-Cbl |
|
1 |
3401(sh), 3350(s), 3159(s) |
1668(vs) |
1668(vs), 1574(s) |
2135(w) |
1216(m) |
|
3 |
3421(s), 3202(sh) |
|
1667(vs), 1574(s), 1719(sh, w) |
2136(vw) |
|
|
4 |
3400(s), 3202(sh) |
|
1667(vs), 1573(m), 1719(s) |
2136(vw) |
|
The IR spectra of compounds 3 and 4 show almost all the absorption bands characteristic for compound 1, except for the band at 1216 cm–1 which refers to ν(C–O) of the free carboxyl group (Fig. 3, Table 1). Furthermore, the IR spectrum of compound 1 displays strong absorption bands at 3350 cm–1 (with a shoulder at 3401 cm–1) and 3195 cm–1, whereas the IR spectra of compounds 3 and 4 contain only the broad bands at about 3400 cm–1, which confirms the assignment of the mentioned bands to stretching vibrations of the H-bonded OH groups in compound 1. The IR spectrum of 1 demonstrates a strong absorption band at 1668 cm–1. The latter is observed also in the IR spectra of products 3 and 4 and can be attributed to the stretching vibrations of the С=О bond in the cobalamin moiety.. The IR spectrum of compound 3 in the range of carbonyl vibrations displays, along with the absorption band at 1668 cm–1, a weak shoulder at 1719 cm–1, which can be attributed to the C=O stretching vibrations of the ester group in the fullerene moiety. A difference in the electronic structures of compounds 3 and 4 consists in the mode of attachment of complex 1 to the fullerene core. In the case of compound 3, where complex 1 is directly attached to the fullerene moiety, the carbonyl group is conjugated with the С60 core. As a result, the absorption band of the C=O bond stretching appears to be shifted to the low-frequency region and coincides with ν(С=О) of the cobalamin moiety. This conclusion is in accordance with the appearance of a strong band at 256 nm in the electronic spectrum of compound 3 (Fig. 4). When the complex is attached to the fullerene core through the ethylene unit as in compound 4, there is no conjugation and the ester group does not differ from that in the fullerene moiety. As a result, the IR spectrum shows a strong band at 1719 cm–1, and the electronic spectrum demonstrates only a weak band at 252 nm.
The resulting data are in good agreement with the electronic spectra of these compounds (Fig. 4). It is well known [17] that the UV-vis spectra of compounds containing corrin ligands show characteristic bands at 360 and 540 nm, which correspond to the transitions of the corrin chromophore. The UV-vis spectra of compounds 1, 3 and 4 display all the bands that evidence the presence of the corrin ligand both in the starting and resulting compounds.
Experimental
General remarks
The IR spectra in the range of 4000–400 cm–1 were recorded with a Bruker Tensor 37 FTIR spectrometer for KBr pellets. The UV-vis spectra in the wavelength range of 220–700 nm were registered with a Jasco V-780 spectrophotometer (Japan) with the scanning rate of 400 nm/min and the scanning step of 0.5 nm for 0.05 M aqueous solutions at 23 °С. The CD spectra were recorded with a SKD-2 unit (combined production of IMB RAS and IS RAS) in the wavelength range of 200–600 nm at 23 °С with the resolution of 3 nm, the acquisition of 2.4 s, and the scanning rate of 35 nm/min. All the measurements were carried out in 1 cm quartz cells. The intensity of CE extreme points in the CD spectra was calibrated using an aqueous solution of d-camphorsulfonic acid. The dichroism magnitudes in all figures are presented as ΔА (the values expressing differences in the light absorbance, which correspond to the right and left circular polarizations).
Compounds 1 and 2 were synthesized according to the published procedures (see Refs. [2] and [9], respectively).
Syntheses
Complex CN-Cbl-C(O)-C60NH(CH2)5COOMe, 3. A solution of compound 1 (49.4 mg, 36.4 μmol) in DMF (5 mL) was added to a solution of thionyl chloride (6.5 mg, 54.6 μmol). The resulting mixture was refluxed for 2 h. The excess of thionyl chloride was removed under vacuum. Then a solution of compound 2 (47.2 mg, 54.6 μmol) in pyridine (5 mL) was added, and the mixture was stirred at room temperature for 8 h and left overnight. A solution was dialyzed against water. The excess of insoluble compound 2 precipitated, whereas unreacted complex 1 moved into a dialysis bag, staining an aqueous phase into a pink color. The dialysis was carried out for 2 days till complete decoloration of dialysis water; the mixture from the dialysis bag was extracted and filtered to remove the precipitate of compound 2 to give an aqueous solution of 3 almost in a quantitative yield.
Complex CN-Cbl-C(O)OCH2CH2-C60NH(CH2)5COOMe 4. A solution of 2-chloroethanol (2.9 mg, 36.4 μmol) and DCC (9.1 mg, 43.7 μmol) was added to a solution of compound 1 (49.4 mg, 36.4 μmol) in DMF (5 mL). The resulting mixture was stirred at room temperature for 2 days. Then, a solution of compound 2 (47.2 mg, 54.6 μmol, 1.5-fold excess) in pyridine (5 mL) was added, and the resulting mixture was stirred at room temperature for 8 h and left overnight. Compound 4 was isolated almost in a quantitative yield according to the above-described procedure.
Conclusions
To sum up the results presented, we showed the possibility to attach a fullerene core of amino acid derivative 2, HC60NH(CH2)5COOMe, to the carboxyl group of complex e-COOH-Cbl-CN 1, which afforded a new type of biologically relevant mixed cobalamin–fullerene complexes 3 and 4.
Acknowledgements
This work was supported by the Russian Foundation for Basic Research (project no. 11-04-01245).
References
1. Bioorganometallics: Biomolecules, Labeling, Medicine, G. Jaouen (Ed.), Weinheim, Wiley, 2006.
2. M. E. Vol'pin, I. Ya. Levitin, S. P. Osinsky, Proc. 3rd Eur. Conf. Bio-Inorg. Chem., Noordwijkerhout, Netherlands, 1996, P2.
3. M. E. Vol'pin, G. N. Novodarova, J. Mol. Catal., 1992, 74, 153–162. DOI: 10.1016/0304-5102(92)80232-6
4. M. E. Vol'pin, N. Yu. Krainova, I. Ya. Levitin, Z. Ya. Mityaeva, G. N. Novodarova, V. K. Oganezov, A. A. Pankratov, V. I. Chissov, R. I. Yakubovskaya, Ross. Khim. Zh., 1998, 116–127.
5. M. E. Vol'pin, D. G. Knorre, G. N. Novodarova, Dokl. Akad. Nauk SSSR, 1988, 298, 363.
6. M. E. Vol'pin, G. N. Novodarova, N. Yu. Krainova, N. F. Krynetskaya, V. G. Metelev, Z. A. Shabarova, Dokl. Akad. Nauk SSSR, 1991, 317, 650.
7. V. M. Belkov, N. F. Krynetskaya, E. M. Volkov, Z. A. Shabarova, N. Yu. Krainova, G. N. Novodarova, M. E. Vol'pin, Bioorg. Khim., 1995, 21, 446.
8. S. J. Lippard, in: Bioinorganic Chemistry, I. Bertini, H. B. Gray, S. J. Lippard, J. S. Valentine (Eds.), Mill Valley, CA (USA), Univ. Sci. Books, 1994, p. 505.
9. V. S. Romanova, V. A. Tsyryapkin, Yu. I. Lyakhovetsky, Z. N. Parnes, M. E. Vol'pin, Russ. Chem. Bull., 1994, 43, 1090–1091. DOI: 10.1007/BF01558092
10. A. I. Kotel'nikov, V. S. Romanova, G. N. Bogdanov, N. P. Konovalova, O. I. Pisarenko, R. A. Kotel'nikova, I. I. Faingol'd, E. S. Frog, Yu. N. Bubnov, M. I. Davydov, S. M. Aldoshin, RF 2462473, 2007.
11. I. I. Faingol'd, R. A. Kotel'nikova, N. P. Konovalova, G. N. Bogdanov, V. S. Romanova, Ezheg. Inst. Problem Khim. Fiz. RAN, 2011, 8, 145–154.
12. R. Taylor, D. R. M. Walton, Nature, 1993, 363, 685–693. DOI: 10.1038/363685a0
13. T. Yu. Dolinina, V. B. Luzhkov, Russ. Chem. Bull., 2012, 61, 1631–1634. DOI: 10.1007/s11172-012-0218-z
14. V. B. Luzhkov, V. S. Romanova, A. I. Kotelnikov, Russ. Chem. Bull., 2014, 63, 567–571. DOI: 10.1007/s11172-014-0474-1
15. M. F. Gillen, R. E. Williams, Can. J. Chem., 1975, 53, 2351–2353. DOI: 10.1139/v75-329
16. N. V. Abramova, K. K. Babievskii, S. M. Peregudova, A. S. Peregudov, V. I. Sokolov, Russ. Chem. Bull., 2011, 60, 2010–2013. DOI: 10.1007/s11172-011-0305-6
17. H. A. O. Hill, J. M. Pratt, R. J. P. Williams, Proc. R. Soc., 1965, A288, 362.





