2019 Volume 2 Issue 1
|
|
INEOS OPEN, 2019, 2 (1), 25–28 Journal of Nesmeyanov Institute of Organoelement Compounds DOI: 10.32931/io1904a |
|
New Effective Method for the Synthesis of 1-Alkoxy(organo)silylmethyl-o-carboranes from Bromomagnesiummethyl-o-carborane and Organoalkoxysilanes
Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, Moscow, 119991 Russia
Corresponding author: G. D. Markova, e-mail: mgaly@yandex.ru
Received 28 April 2018; accepted 5 October 2018
Abstract

An efficient method for the synthesis of 1-alkoxy(organo)silylmethyl-o-carboranes is developed starting from 1-bromomagnesiummethyl- and 1-bromo-magnesiummethyl-2-methyl-o-carboranes as key precursors. The method provides 1-methoxy(dimethyl)silylmethyl-, 1-ethoxy-(dimethyl)silylmethyl-, 1-diethoxy(methyl)silylmethyl-, 1-tri-ethoxysilylmethyl-, 1-methoxy(dimethyl)silylmethyl-o-carboranes and 1-diethoxy(methyl)silylmethyl-2-methyl-o-carborane.
Key words: Grignard reagent, bromomagnesiummethyl-о-carborane, 1-bromomagnesiummethyl-2-methyl-о-carborane, dimethyldimethoxysilane, dimethyldiethoxysilane, tetraethoxysilane.
Introduction
Earlier we have shown that alkoxy(organo)silylmethyl-o(m)-carboranes and 1,2(7)-bis[alkoxy(organo)silylmethyl]-o(m)-carboranes can be obtained by the reactions of mono- and dilithium-o(m)-carboranes with chloromethyl(organo)-alkoxysilanes [1, 2]. In these compounds, R3Si–CH2CB10H10C– fragment exhibits high thermal stability and stability to the action of air moisture as well as acidic and alkaline reagents. These results appeared to be contrary to the previously reported data about carboranesiliconorganic compounds and organosiloxane polymers bearing R3Si–CB10H10C– and R3Si–CH2CH2CB10H10C– fragments [3]. The former readily decompose at high temperatures under action of moisture, acidic and alkaline reagents, resulting in carborane and organosiloxanes. The latter display low thermal stability due to the presence of an ethylene unit between the silicon and carbon atoms in the carborane core. At present, much attention is drawn to the synthesis of 1,7-bis[bromo(organo)silylmethyl]-carborane monomers [4] and investigation of the thermal and thermooxidative stability of organosiloxanes obtained from these monomers and bearing 1,7-bis(dimethylsilylmethyl)-m-carborane units [5].
Of particular interest are also the works on the synthesis and properties of new polydimethylsiloxanes with terminal m-car-boranyl(propyl)silyl groups [6], new multifunctional carboranylpropyl-substituted octasilsesquioxanes [7], oligocarboranesiloxanes obtained by transesterification of bis(triethoxysilylpropylamide)-m-carborane with bis(hydroxy-methyl)-o-carborane, bis(hydroxymethyl)-m-carborane, and ethylene glycol [8], and also a new method for the preparation of bis(alkoxysilylmethyl)carboranes from bis(bromo-magnesiummethyl)carboranes [9].
Recently, we have studied the reactions of halogenmagnesiummethyl-m-carboranes with organoalkoxysilanes and chlorosilanes and suggested a new method for the synthesis of 1-alkoxy(organo)silylmethyl-m-carboranes from chloromagnesiummethyl-m-carborane and organoalkoxysilanes as well as a new method for the preparation of 1,7-bis-[methoxy(dimethyl)silylmethyl]-m-carborane and 1,7-bis-[chloro(dimethyl)silylmethyl]-m-carborane from 1,7-bis-[bromomagnesiummethyl]-m-carborane, dimethyldimethoxy-silane and dimethyldichlorosilane [10]. It seemed interesting to develop a synthetic approach to 1-alkoxy(organo)silylmethyl-o-carboranes using 1-bromomagnesiummethyl-o-carborane and organoalkoxysilanes as key precursors. Note that earlier 1-alkoxy(organo)silylmethyl-o-carboranes were obtained by the reactions of 1-lithium-o-carborane with carbofunctional chloromethyl(organo)alkoxysilanes [1, 2]. In this work, we renounced the use of organochlorosilanes due to their high toxicity.
Herein, we report on the results of investigation of the reactions of 1-bromomagnesiummethyl-o-carborane and 1-bromomagnesiummethyl-2-methyl-o-carborane with dimethyldimethoxysilane, dimethyldiethoxysilane, methyltriethoxysilane, and tetraethoxysilane.
Results and discussion
The reactions of 1-bromomagnesiummethyl-о-carborane with an excess of dimethyldimethoxysilane, dimethyldiethoxysilane, methyltriethoxysilane, or tetraethoxysilane in diethyl ether readily afforded 1-methoxy(dimethyl)silylmethyl-о-carborane (I), 1-ethoxy-(dimethyl)silylmethyl-о-carborane (II), 1-diethoxy(methyl)-silylmethyl-о-carborane (III), and 1-triethoxysilylmethyl-о-carborane (IV), respectively (Scheme 1).

Scheme 1. Reactions of 1-bromomagnesiummethyl-о-carborane with organoalkoxysilanes in diethyl ether.
In turn, the above-mentioned Grignard reagent was obtained by the reaction of 1-bromomethyl-о-carborane with magnesium in diethyl ether according to the published method (Scheme 2) [10].
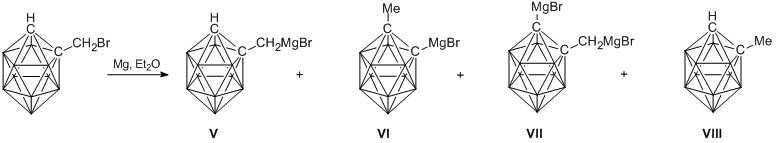
Scheme 2. Synthesis of the Grignard reagent.
The main reaction product appeared to be target compound V. Isomeric monomagnesium compound VI, dimagnesium derivative VII, and 1-methyl-o-carborane VIII were formed only in small amounts. The ratio of products V, VI, VII, and VIII slightly varied depending on the reaction conditions, such as the amounts of magnesium and ether as well as the rate of addition of 1-bromomethyl-o-carborane. Thus, at the molar ratio of 1-bromomethyl-o-carborane: magnesium = 1:2, the content of 1-bromomagnesiummethyl-o-carborane in the reaction mixture, determined by the bromination method [10], composed 88–92%. A reduction of the amount of magnesium led to a slight increase in the yields of compounds VI, VII, and VIII.
The yields of alkoxy(organo)silanes I–IV in the reactions of 1-bromomagnesiummethyl-o-carborane with the corresponding silicon-containing reagents composed 80%.
Earlier using m-carborane derivatives as examples, it was shown that the application of disubstituted carboranes allow one to avoid the isomerization and re-metalation processes and, as a result, to increase the yield of the target Grignard reagent [10]. In the present work, we showed that the reaction of 1‑bromomethyl-2-methyl-o-carborane with magnesium in diethyl ether gives 1-bromomagnesiummethyl-2-methyl-o-carborane in 95% yield. The reactions of this Grignard reagent with dimethyldimethoxysilane or methyltriethoxysilane afforded 1-methoxy(dimethyl)silylmethyl-2-methyl-o-carbonane (IX) and 1-diethoxy(methyl)silylmethyl-2-methyl-o-carborane (X), respectively (Scheme 3). The yields of compounds IX and X reached 85%.

Scheme 3. Synthesis of 1-methoxy(dimethyl)silylmethyl-2-methyl-
and 1-diethoxy(methyl)silylmethyl-2-methyl-о-carboranes.
The physicochemical constants, the data of elemental analyses, and the IR and NMR spectroscopic data for compounds I–IV, IX, and X are given in Tables 1 and 2.
Table 1. Physicochemical constants and elemental analyses for compounds I–IV, IX, and X
|
Compound |
Molecular |
Bp, °C |
nd20 |
Anal. Found and Calcd, % |
|||
|
C |
H |
B |
Si |
||||
|
I |
C6H22B10OSi |
132–134 40–41a |
– |
29.70 29.24 |
9.10 8.97 |
44.10 43.88 |
11.78 11.40 |
|
II |
C7H24B10OSi |
154–156 |
1.5055 |
32.43 32.27 |
9.07 9.28 |
42.05 41.50 |
11.74 10.78 |
|
III |
C8H26B10O2Si |
150–152 |
1.5320 |
33.38 33.07 |
8.97 9.02 |
37.75 37.21 |
9.23 9.66 |
|
IV |
C9H28B20O3Si |
154–156 |
1.5530 |
34.33 33.69 |
8.13 8.87 |
33.93 33.70 |
8.62 8.75 |
|
IX |
C7H24B10O2Si |
135–137 |
1.5290 |
32.15 32.27 |
9.17 9.28 |
41.0 41.50 |
10.32 10.78 |
|
X |
C9H28B10O2Si |
140–143 |
1.5350 |
36.12 35.49 |
9.12 9.27 |
35.73 35.50 |
9.17 9.22 |
a Mp, °C
Table 2. IR and 1H NMR spectroscopic data for compounds I–IV, IX, and X
|
Compound |
IR (ν, сm–1) |
1H NMR (CDCl3, δ, ppm) |
|||||||
|
B–H |
Si–CH2 |
CH3 |
CH2 |
OCH3 |
OCH2CH3 |
OCH2CH3 |
Ccb-CH3 |
CHcb |
|
|
I |
2600 |
2980 2900 |
0.47 |
2.14 |
3.72 |
– |
– |
– |
4.15 |
|
II |
2600 |
2980 2900 |
0.30 |
2.00 |
– |
1.40 |
3.90 |
– |
4.07 |
|
III |
2600 |
2980 2900 |
0.28 |
2.03 |
– |
1.60 |
4.25 |
2.43 |
– |
|
IV |
2600 |
2980 2900 |
– |
1.88 |
– |
1.28 |
3.90 |
– |
4.07 |
|
IX |
2600 |
2980 2900 |
0.25 |
1.62 |
3.42 |
– |
– |
2.07 |
– |
|
X |
2600 |
2980 2900 |
0.29 |
2.05 |
– |
1.62 |
4.28 |
2.41 |
– |
Experimental
General remarks
The experiments were concerned with 1-bromomethyl-о-carborane (mp: 47–49 °C, hexane), 1-bromomethyl-2-methyl-о-carborane (mp: 125–127 °C, hexane), dimethyldimethoxysilane (bp: 80 °C, nd = 1.3708), dimethyldiethoxysilane (bp: 111 °C, nd = 1.3839), methyltriethoxysilane (bp: 151 °C, nd = 1.3861), tetraethoxysilane (bp: 166 °C, nd = 1.3837), and magnesium filings. Diethyl ether was distilled over LiAlH4. All the reactions with magnesium derivatives of o-carborane were carried out in an atmosphere of dry nitrogen. GLC was carried out on 2 m long columns filled with 10% E-301 silicone elastomer on a chromaton in the temperature range of 150–180 °C using helium as a carrier gas. TLC analysis was carried out on glass plates with alumina (eluent: petroleum ether, staining: iodine vapor). NMR spectra were recorded on a Bruker Avance 400 spectrometer in CDCl3 using HMDS as an internal reference. IR spectra were recorded on a Specord M80 spectrophotometer (Germany) in the frequency range of 4000–400 cm−1 in KBr pellets.
Syntheses
Reaction of 1-bromomethyl-о-carborane with magnesium in diethyl ether.
Magnesium filings (1.80 g, 0.078 mol) in 50 mL of diethyl ether were activated by addition of several drops of 1,2-dibromoethane. Then a solution of 1-bromomethyl-о-carborane (7.70 g, 0.039 mol) in 50 mL of diethyl ether was added. The reaction mixture was refluxed for 1 h. To control the reaction course and determine its completion, samples of the reaction mixture were hydrolyzed and analyzed by GLC. The resulting solution of 1-bromomagnesiummethyl-o-carborane was filtered to remove unreacted magnesium. The bromination was carried out according to the published procedure [10]. The GLC analysis showed that the resulting mixture contained
1-bromomethyl-o-carborane (88–92%), 1-bromo-2-methyl-o-carborane (4%), 1-bromo-2-bromomethyl-o-carborane (1%), and 1-methyl-o-carborane (3%). These compounds were used as standards for GLC analysis. The data obtained evidence that the content of 1-bromomagnesiummethyl-o-carborane (V) in the reaction mixture was 88–92%. The contents of 1-methyl-2-bromomagnesium-o-carborane, 1-bromomagnesium-2-bromomagnesiummethyl-o-carborane, and 1-methyl-o-carborane composed 4%, 1%, and 3%, respectively.
Reaction of 1-bromomethyl-2-methyl-о-carborane with magnesium in diethyl ether.
Magnesium filings (1.20 g, 0.049 mol) in 50 mL of diethyl ether were activated by addition of several drops of 1,2-dibromoethane. Then a solution of 1-bromomethyl-2-methyl-o-carborane (5.02 g, 0.02 mol) in 50 mL of diethyl ether was added. The reaction mixture was refluxed for 1 h. To control the reaction course and determine its completion, samples of the reaction mixture were hydrolyzed and analyzed by GLC. The resulting solution of 1-bromomagnesium-2-methyl-o-carborane was filtered to remove unreacted magnesium. The bromination was carried out according to the published procedure [10]. The content of 1-bromomagnesiummethyl-2-methyl-o-carborane in the reaction mixture was 95%.
Reactions of 1-bromomagnesiummethyl-o-carborane with dimethyldimethoxysilane, dimethyldiethoxysilane, methyltriethoxysilane, and tetraethoxysilane.
A solution of 1-bromomagnesiummethyl-o-carborane (13.00 g, 0.05 mol) in 70 mL of diethyl ether was added dropwise to a stirred solution of (18.00 g, 0.15 mol) in 70 mL of diethyl ether. The reaction mixture was refluxed for 3 h. After cooling to room temperature, 1-methyl-o-carboborane (IV) was removed from the resulting mixture under vacuum to give 9.80 g of 1-methoxy(dimethyl)silylmethyl-o-carborane (compound I). Yield: 80%.
Analogously, the reactions of 1-bromomagnesiummethyl-o-carborane (13.00 g, 0.05 mol) with dimethyldiethoxysilane (22.24 g, 0.15 mol), methyltriethoxysilane (26.70 g, 0.15 mol), or tetraethoxysilane (41.66 g, 0.20 mol) afforded 10.40 g of compound II, 11.60 g of compound III, and 12.80 g of compound IV, respectively. In all cases the yields of the target products comprised 80%. The physicochemical characteristics of compounds I–IV are given in Tables 1 and 2.
Reactions of 1-bromomagnesiummethyl-2-methyl-о-carborane with dimethyldimethoxysilane and methyltriethoxysilane.
A solution of 1-bromomagnesiummethyl-2-methyl-o-carborane (6.68 g, 0.025 mol) in 70 mL of diethyl ether was added dropwise to a stirred solution of dimethyldimethoxysilane (9.00 g, 0.015 mol) in 70 mL of diethyl ether. The reaction mixture was refluxed for 3 h. After cooling to room temperature, an excess of dimethyldimethoxysilane was removed on a rotary evaporator to give 5.52 g of 1-methoxy(dimethyl)silylmethyl-2-methyl-o-carborane (compound IX). Yield: 85%.
Analogously, the reaction of 1-bromomagnesiummethyl-2-methyl-o-carborane (6.68 g, 0.025 mol) with methyltriethoxysilane (13.40 g, 0.075 mol) afforded 6.50 g of 1-diethoxy(methyl)silylmethyl-2-methyl-o-carborane (X). Yield: 85%. The physicochemical characteristics of compounds IX and X are given in Tables 1 and 2.
Conclusions
To summarize the results presented, we showed the possibility of obtaining 1-methoxy(dimethyl)silylmethyl-, 1-ethoxy(dimethyl)silylmethyl-, 1-diethoxy(methyl)silylmethyl-, and 1-triethoxysilylmethyl-o-carboranes from 1-bromo-magnesiummethyl-o-carborane, and dimethyldimethosilane, dimethyldiethoxysilane, methyltriethoxysilane, and tetraethoxysilane in 80% yields. It was also found that the reactions of 1-bromomagnesiummethyl-2-methyl-o-carborane with dimethylsilane or dimethoxysilane afford 1-methoxy-(dimethyl)silylmethyl-2-methyl- or 1-diethoxy(methyl)silyl-2-methyl-o-carboranes in 85% yields.
Acknowledgements
The authors are grateful to the contribution of Center for molecular composition studies of INEOS RAS.
References
- V. N. Каlinin, B. А. Izmaylov, А. А. Каzantzev, L. I. Zakharkin, K. A. Andriyanov, Dokl. Akad. Nauk SSSR, 1979, 246, 616–620.
- V. N. Каlinin, B. А. Izmaylov, А. А. Каzantzev, V. D. Myakushev, А. А. Zhdanov, L. I. Zakharkin, J. Organomet. Chem., 1981, 216, 295–320. DOI: 10.1016/S0022-328X(00)85813-1
- R. N. Grimes, Carboranes, 2nd ed., Acad. Press, 2011, p. 541.
- X.-J. Han, H.-F. Fei, B.-Z. Liu, Y.-X. Tan, X.-Z. Zhang, Z.-M. Xie, Z.-J. Zhang, RSC Adv., 2015, 5, 76079–76082. DOI: 10.1039/c5RA13344K
- P. Low, X. Zhang, B. Liu, X. Gao, Z. Zhang, Z. Xie, Polym Degrad. Stab., 2017, 144, 304–314. DOI: 10.1016/j.polymdegradstab.2017.08.029
- A. A. Anisimov, A. V. Zaytsev, V. A. Ol'shevskaya, M. I. Buzin, V. G. Vasil'ev, K. L. Boldyrev, O. I. Shchegolikhina, V. N. Kalinin, A. M. Muzafarov, Mendeleev Commun., 2016, 26, 524–526. DOI: 10.1016/j.mencom.2016.11.022
- A. A. Anisimov, V. A. Ol'shevskaya, R. A. Novikov, A. A. Korlyukov, M. I. Buzin, O. I. Shchegolikhina, V. N. Kalinin, A. M. Muzafarov, J. Organomet. Chem., 2016, 822, 1–4. DOI: 10.1016/j.jorganchem.2016.08.011
- B. A. Izmaylov, V. A. Vasnev, A. S. Peregudov, G. D. Markova, E. N. Rodlovskaya, V. I. Bregadze, J. Organomet. Chem., 2017, 844, 16–29. DOI: 10.1016/j.jorganchem.2017.05.038
- B. I. Izmaylov, Y. P. Bai, V. A. Vasnev, G. D. Markova, J. Organomet. Chem., 2018, 867, 220–223. DOI: 10.1016/j.jorganchem.2017.12.012
- B. A. Izmaylov, V. A.Vasnev, G. D. Markova, Inorg.Chim. Acta, 2018, 471, 475–480. DOI: 10.1016/j.ica.2017.11.056





