2019 Volume 2 Issue 1
|
|
INEOS OPEN, 2019, 2 (1), 1–8 Journal of Nesmeyanov Institute of Organoelement Compounds DOI: 10.32931/io1901r |
|
Tribochemical Activity in Polymers
Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, Moscow, 119991 Russia
Corresponding author: A. P. Krasnov, e-mail: krasnov@ineos.ac.ru
Received 23 November 2018; accepted 6 February 2019
Abstract

Triboactivity of polymers is studied as a chemical process that significantly affects their friction. The examples are provided which demonstrate the tribochemical activity in linear amorphous polymers, such as polyphenylquinoxalines, polyacrylonitrile, crystalline aliphatic polyamides and polyaramids as well as polyamidoimide binder. The process of self-organization in a heat-resistant composite material based on a phenol formaldehyde polymer is analyzed in detail.
Key words: tribochemical activity and stability, intermolecular and intramolecular reactions, self-organization of polymer composites, heat-resistant polymers.
1. Terms and definitions
Dry friction is often called as the friction of unlubricated surfaces that arises in the absence of lubricants and impurities between the surfaces.
Coefficient of sliding friction (f) is the ratio of the tangential force, which is spent to overcome the resistance to a relative displacement of two bodies, to the load that compresses the contacting bodies.
Amplitude of vibration of the friction coefficient is defined as the difference between the maximum and minimum values of the friction coefficient.
Coefficient of mutual overlapping (k) is the ratio of areas of bodies in friction contact: the area of a counterbody (S1) to that of a tested sample (S2), k = S1/S2. Depending on the test methodology, it changes from 1 at the equal areas of the tested bodies to extremely low values in the case of a scheme "a steel ball against a polymer sample".
Friction tests are carried out under isothermal conditions at the constant load and rate.
Thermofriction tests are carried out at the constant load and rate upon changing the friction temperature owing to external heating. The test results are presented in the form a thermofriction dependence of the friction coefficient on the temperature. The tests are usually performed at k = 1 and the heating rate of 5 or 10 °С/min.
Friction heat stems from heating of rubbing bodies that depends on the coefficient of friction. At the reduced friction coefficient (~0.30–0.35) in antifriction materials under consideration and the constant rate, the temperature of a steel sample increases depending on the load and the coefficient of mutual overlapping.
Third body is a term which is often used in tribological literature. It is defined as the region of friction contact that includes the surfaces of polymer and steel samples as well as wear debris.
Secondary structures are formed on the surface of a polymer sample mainly depending on the temperature and duration of friction and represent only a part of the processes that take place upon friction of polymers in third body.
Technological systematization of polymers, which are used in friction joints, divides them into antifriction (the friction coefficient under standardized conditions reaches up to 0.35) and friction ones (the friction coefficient is over 0.40).
According to these parameters, some of the polymers are widely used in antifriction heat-resistant joints of dry friction (PEEK, PPS) and joints of dry friction (poly(para-aramid) (Kevlar), phenol formaldehyde polymers).
Heterochain parameter (HCP) in aliphatic polyamides is the ratio of the number of amide groups to that of the methylene units (%).
2. Effect of tribochemical reactions on friction properties of polymers
The first report on common dependences between the chemical structure of different classes of polymers and their tribological properties based on their tribochemical behavior was published in Journal of Friction and Wear in 2002 [1].
The systematization of polymers of tribological application consists in their division into tribochemically active (triboactive) and tribochemically stable (tribostable) polymers based on their chemical structure and tribochemical activity. The tribochemical activity means an increase and significant growth of amplitude of vibrations of the polymer friction coefficient, which are connected, as a rule, with the active tribochemical processes [2] and lead to a considerable increase of wear. Friction of tribochemically active polymers causes substantial friction heating.
To mitigate an effect of a sharp increase of the friction heat, graphite is often introduced into polymers. Although graphite is considered to be self-lubricating filler, we showed that during friction it performs mainly separating antiadhesion function toward the counterbody steel [3, 4]. This leads to a decrease and stabilization of the polymer friction coefficient owing to a laminated structure of graphite, but does not affect the temperature limits of variation of the friction coefficient. The intensity of tribochemical processes also reduces. The effect of graphite on polymer friction strongly differs from that of molybdenum disulfide. Recently, the lubricating properties of graphite were analyzed using a special technique which included X-ray photoelectron spectroscopy (XPS) [5, 6]. The introduction of the superior self-lubricating filler markedly affects a whole set of properties. The characteristics of carbon fillers are affected by the temperature of thermal processing, but actually their introduction does not improve the lubricating properties.
Among numerous factors of friction instability (tribochemical activity) of polymers, of particular attention are the chemical processes that take place during friction and are connected with the reactions of polymer functional groups [7]. Their role was demonstrated in tribological investigations of two typical linear polyheteroarylenes, namely, polyphenylquinoxalines PPQ-1 and PPQ-3 and their copolymer PPQ-3. The structures of these polymers are presented in Fig. 1. The terminal groups of the polyphenylquinoxalines are electron-donating NH2 groups. The introduction of electron-withdrawing carbonyl groups into macromolecules of the copolyphenylquinoxaline led to an increase in the friction activity already at room temperature. It was found that the friction promotes the reaction of carbonyl groups with terminal amino groups, resulting in more stable azomethine bonds (Scheme 1).

PPQ-1 Mw = 46·103, Тs = 120 °С

a
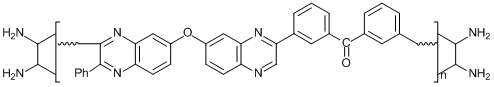
PPQ-2 Mw = 52·103, Ts = 220 °С

b
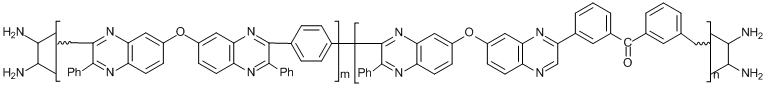
PPQ-3 Mw = 69·103, m:n = 1:1, Тs = 150 °С

c
Figure 1. Thermofriction curves of PPQ-1 (а), PPQ-2 (b) and PPQ-3 (c) (v = 0.1 m/s, P = 0.2 MPa). The polymer samples contained 60 wt % of graphite. Zones: stable friction (A), softening (B), formation of the secondary surface (C). Ts is the softening point of a sample.

Scheme 1. Reaction of carbonyl and amine groups upon friction, resulting in azomethine bonds.
Figure 1 depicts the thermofriction curves of all the samples explored. The curves show three characteristic zones of the friction coefficient variation: zone of stable friction (А), zone of polymer softening (B), and zone of the samples with the secondary surfaces (C). For PPQ-1, which does not form cross-linkages during processing, the softening process starts already at ~130 °С. The friction coefficient of PPQ-1 has the lower value than those of reactive polymers PPQ-2 and PPQ-3 bearing C=O bonds. This makes it a typical representative of cast heat-resistant thermoplastics, since the formation of cross-linkages would inevitably increase the friction coefficient of the polymer. Reactive polymers PPQ-2 and PPQ-3 feature higher starting friction coefficients. Presumably, the cross-linkages are formed in their structures already during the processing stage. A stepwise character of the change of friction coefficient of copolymer PPQ-3 in zone А is likely to be connected with the formation of cross-linkages, which intensifies at 120 °С and continues up to 220 °С. This results in a cross-linked, more heat-resistant structure, which is evidenced by the higher friction coefficient on passing to the friction of the resulting secondary structure (zone С, Fig. 1b). The most stable change of the friction coefficient upon temperature rise was observed for polymer PPQ-2 which has the softening point of about 220 °С and contains the maximum amount of C=O ketone groups in an elementary unit.
Hence, the friction under mechanical impact and temperature rise is likely to cause the formation of new azomethine bonds in rubbing layers of the surfaces of filled polymers, which results in more heat-resistant structures.
In the above-mentioned examples, the friction promoted the formation of network structures in the polymers. To define the effect of such network structures on the friction properties of cross-linked polymers, reactoplastics with variable degree of cure were studied. The investigations were concerned with polyamidoimide (PAI) samples obtained from bis(maleimido)diphenylmethane (BMI) (Scheme 2) [8–10].

Scheme 2. Synthesis of the cross-linked polymer based on bis(maleimido)diphenylmethane.
The thermofriction tests were carried out using two samples of the polymers. In the first case, the degree of conversion of the double bonds was 85%, which led to the formation of highly cross-linked rigid structures; in the second case, the conversion degree was 60%.
Figure 2 shows the thermofriction curves of the samples explored. The polymerization degree strongly affects the shapes of the resulting dependences. At 60% conversion, the curve almost does not reveal the zones of stable f. As the conversion degree increases to 85%, the stage of unstable friction coefficient completes at 150 °С.

Figure 2. Thermofriction curves of polyamidoimide (PAI) coatings based on bis(maleimide) filled with a mixture of graphite and molybdenum disulfide. The total content of the fillers was 60 wt %. Testing conditions: v = 0.3 m/s, P = 0.1 MPa, duration 35 min, heating rate 10 deg/min.
(1) Сoating based on PAI with 60% conversion of the double bonds;
(2) coating based on PAI with 85% conversion degree;
(3) repeated test with sample 1 after cooling.
Of particular attention is thermofriction curve 3 (Fig. 2), which corresponds to the repeated test of sample 1 with the conversion degree of 60%. It does not reveal the vibration change of the friction coefficient. The variation of f between the starting and final values is insignificant. Presumably, the thermofriction impact led not only to an increase in the conversion degree of the polymer double bonds, but also to purification of the sample surface from unstable chemical groups.
The results of the above-mentioned experiments allow one to conclude that the tribochemical activity (triboactivity) of network polymers obtained from reactive oligomers is defined both by the chemical structures of the components and by the flexibility of a fragment between the adjacent cross-links. The retention of reactive functional groups in the structure of a sparsely cross-linked polymer facilitates their interaction during friction, which results in the formation of a new and, what is important, more heat-resistant surface structure that cannot be achieved in the sample of a highly cross-linked polymer.
The presence of functional groups in heat-resistant polymers does not ensure the formation of network structures during friction. For example, the investigation of polyacrylonitrile (PAN) and its compositions with MoS2 and graphite showed that the high reactivity of the nitrile groups predetermines the multiple possibilities of chemical transformations during friction of polymer nitriles, especially in air [11]. In this atmosphere upon thermal processing under static conditions, there are observed, as a rule, intensive gas release, reduction of the amount of nitrile groups, appearance of imine and carbonyl groups, naphthyridine rings, and polyconjugated systems, and gradual conversion of polyacrylonitrile into a ladder-type cyclic polymer which idealized structure is presented in Scheme 3 [12].

Scheme 3. Conversion of PAN to a ladder cyclic polymer at elevated temperatures.
Starting PAN features the high friction coefficient that has considerable amplitude of vibrations in the range from 0.5 to 1.0 even at room temperature, well before the glass-transition point (Fig. 3). At the higher temperature, friction occurs in a seizing mode. This behavior of PAN during friction characterizes it as a tribochemically active polymer, which is associated mainly with the predominant contribution of chemical processes into the value and character of the friction coefficient variation. Let us note that the friction of all PAN samples explored, including the filled ones, led to an increase in the oxygen content on the friction surface, which was more pronounced in the sample bearing MoS2 (an increase from 5.2% to 42.4%).

Figure 3. Effect of the temperature on the friction coefficient of PAN filled with 30 wt % of MoS2 (v = 0.5 m/s, P = 0.05 MPa) (1) and thermomechanical curve of the composite (2). The change of the friction coefficient is presented as a range of amplitude of vibrations of this parameter.
Hence, the thermal stability of polymers does not predetermine their tribological properties. The thermofriction stability of triboactive polymers can be achieved owing to the formation of a new, more thermally stable surface during friction.
3. Tribochemical activity in aliphatic polyamides [7]
One of the types of polymers frequently used in friction joints with single or limited lubrication are linear aliphatic polyamides. Note that polyamides find little if any application in dry friction due to their tribochemical activity [13, 14].
Earlier it was shown that the properties of aliphatic polyamides such as heat resistance and thermal stability are defined by the ratio of amide and methylene groups in a polymer unit, the so-called heterochain parameter (HCP). The dependences of thermophysical properties of aliphatic polyamides on this characteristic were described in reference [15]. Table 1 presents the physicomechanical properties of the initial and carbon fiber reinforced polyamides. There is a clear dependence of the polymer characteristics on the chemical structures of macromolecular chains. Thus, aliphatic polyamides appeared to be convenient objects for solution of the problem of correlation between the tribological properties and the chemical structure of a polymer.
Table 1. Physicomechanical properties of the initial polyamides and polyamides reinforced by short carbon fibers
|
Parameter |
Initial polyamides |
Carbon fiber reinforced polyamides (30%) |
||||||
|
PA6 |
PA66 |
PA610 |
PA12 |
PA6 |
PA66 |
PA610 |
PA12 |
|
|
Mp, °C |
220 |
260 |
215 |
178 |
– |
– |
– |
– |
|
Density, g/cm3 |
1.1 |
1.14 |
1.1 |
1.01 |
1.3 |
1.3 |
1.2 |
1.1 |
|
σbend, MPa |
90 |
80 |
45 |
54 |
260 |
200 |
196 |
181 |
|
Еbend, GPa |
2.7 |
3.0 |
1.5 |
1.2 |
17.5 |
18.4 |
14.6 |
11.7 |
|
σtens, MPa a |
55–77 |
80 |
50–60 |
53 |
174 |
162 |
151 |
136 |
|
Tensile elongation, % |
– |
– |
– |
– |
2.3 |
1.9 |
2 |
4.8 |
|
Specific viscosity, А, kJ/m2 b |
10 |
7.5 |
4.9 |
20 |
21.5 |
11 |
16 |
28 |
|
HCP, % |
20 |
20 |
14 |
8 |
– |
– |
– |
– |
a Polymers PA6 and PА610 were tested in parallel using two batches.
b According to the Charpy impact tests with notched (initial polymers) and unnotched (carbon fiber reinforced polymers) samples.
The structural formulae of the polymers under consideration are presented below.
[–NH–(CH2)5–CO–]n PA6
[–NH–(CH2)6–NH–CO–(CH2)4–CO–]n PA66
[–NH–(CH2)11–CO–]n PA12
[–NH–(CH2)6–NH–CO–(CH2)8–CO–]n PA610
CF/PAs are carbon-filled cast polyamides reinforced by short carbon fibers (CF 250 μm).
Since we tried to establish the effect of a heterochain parameter on tribological properties, the reference sample was ultrahigh-molecular-weight polyethylene (UHMWPE, HCP = 0) that withstands friction conditions.
The results of friction tests presented in Fig. 4 show that the introduction of amide bonds into the aliphatic structure of polyethylene facilitates an increase of the tribochemical activity of the polymer and a growth of its friction coefficient. The nature of tribochemical activity of PA is defined by the tribooxidative process involving the carbon atom of alpha-methylene group and resulting in peroxide and hydroperoxide units [16].

Figure 4. Effect of a heterochain parameter (HCP) on the antifriction properties of polyamides and carbon plastics. А is the friction coefficient of UHMWPE (1) and the initial polyamides: PA12 (2), PA610 (3), PA6 (4), and PA66 (5) (P = 0.03 MPa, v = 0.2 m/s). B is the friction coefficient of CF/PAs bearing 30% of short carbon fibers: PA12 (2'), PA610 (3'), PA6 (4'), and PA66 (5') (Р = 0.1 MPa, v = 1 m/s). Note that filled UHMWPE appeared to be inoperable under the chosen conditions.
Reinforcement of polyamides by short carbon fibers leads to the fact that the resulting composites retain the dependence of tribochemical activity on a heterochain parameter during friction. As can be seen from Fig. 4 (curve B), in all compositions, the carbon filler affords almost the same changes in antifriction parameters of carbon fiber reinforced plastics, for example, CF/PA6 and CF/PA66, independent from their structures. It is important to note that the introduction of carbon fibers leads to an increase in the friction coefficient by 0.8f rather than its reduction.
The revealed dependence of tribochemical activity of aliphatic PAs on the number of amide bonds in a macromolecule unit opens the way to solution of practical problems connected with the production of materials featuring optimal tribological properties [17]. The results obtained show that the dependence of the friction coefficient on the heterochain characteristic is valid not only for polyamides, but also for carbon fiber reinforced plastics based on them.
4. Effect of tribochemical activity of polyaramid fibers on friction of organoplastics [18]
It is well known that aliphatic and aromatic polyamides significantly differ in the properties. However, we showed that the tribochemical activity of polymers is manifested also in the case of high-molecular polyaramid fibers [19]. The investigations were concerned with organoplastics based on a phenol formaldehyde resin reinforced by polyaramids (Schemes 4 and 5): para-aramid (Kevlar) [20, 21] and meta-aramid (Nomex) [22]. The samples of the reinforced polymers were prepared so that the reinforcing fibers appeared to be on their surfaces. The production technology included processing of a surface of the pressed sample with a polishing abrasive cloth until removal of a layer of poorly cured resin. As soon as the surface layer was worn out, the friction coefficient sharply increased, which indicated the appearance of reinforcing fibers on the surface. In addition to the mentioned fibers, polyoxadiazole (POD) fibers were studied for comparison [23, 24]. The samples were prepared analogously. The properties of the fibers explored are presented in Table 2.
Table 2. Properties of fibers
|
Fiber |
Crystallinity |
Thermal destruction |
Density, |
Strength, GPa or kN/tex a |
|
Kevlar |
95–99 |
500 |
1.45–1.47 |
2.7–4.5 |
|
Nomex |
61 |
390 |
1. 37–1.38 |
40–50 a |
|
POD |
74 |
460 |
1.42—1.44 |
50–80 a |
a The strength was measured in kN/tex.
The friction coefficient of a polymer binder of organic plastics, phenol formaldehyde (PF) polymer, in the first 10–30 min composed 0.22–0.25, which is essentially lower than the friction coefficient of heat-resistant fibers (0.35–0.55). In this case, the effect of the matrix polymer on tribological behavior of the reinforced samples can be neglected.
Figure 5 depicts the friction dependences of the friction coefficients for the samples explored. Of particular attention is an order of variation of the friction coefficient and amplitude of its vibrations at Рsp = 0.1 MPa: meta-aramid (f ~ 0.9) > para-aramid (f ~ 0.7) > POD fiber (f ~ 0.45). It is also noteworthy that frictions of the first two samples occurred in a seizing mode and had unsteady character with extremely high amplitudes of vibrations.

a

b
Figure 5. Friction of organoplastics based on heat-resistant fibers from polyheteroarylene (PGA) and PF polymer binder against steel: poly(meta-aramid) (1); poly(para-aramid) (2); POD (3) (t = 30 min, v = 0.5 m/s). Conditions: Рsp = 0.1 MPa, k = 1, friction joint: butt ends of two hubs (a); Рsp = 20 MPa, k = 0.1, friction joint: steel ball–plain (b).
At the pressure of 20 MPa, the order of the friction coefficient decrease was the same, but its absolute values markedly changed: meta-aramid (f ~ 0.45) > para-aramid (f ~ 0.36) > POD fibers (f ~ 0.27). In both experiments, a steady character of friction with insignificant vibration amplitude was manifested only by the organic plastic based on POD fibers.
To explain the result obtained, we initiated a comprehensive survey of the samples, which was aimed to establish the effect of different structural factors on the friction of the organic plastics under consideration. It was found that the sequences of variation of crystallinity, physicomechanical properties, and heat resistance do not correlate with the experimentally deduced order of variation of the friction coefficients; therefore, the higher tribological characteristics of the composites based on POD fibers are not stipulated by these parameters. Unfortunately, an attempt to define the relationship between the energy of intermolecular interactions in polyaramids and polyoxadiazoles and their tribological parameters using computational methods failed [25].
Based on the fact that POD fibers do not include tribochemically active amide bonds, we concluded that their tribostability is caused by a chemical structure of the fiber. As a consequence, there cannot be a common correlation between the tribochemically active and tribostable heat-resistant polymers and their friction.
It was interesting to find a reason for the difference in friction coefficients of para- and meta-aramids, which have the same amounts of amide bonds in units.
As can be seen from Scheme 4, the arrangements of macromolecules in crystalline structures of para-aramid and meta-aramid considerably differ [26]. The distance between macromolecules of poly(para-aramid) reaches 4.71 Å owing to para–para addition of amine and acid components or linear arrangement of the macromolecule. For meta-aramid featuring nonlinear arrangement, the distance between adjacent macromolecules increases up to 6.7 Å, which is reflected in weakening of the hydrogen bond energy in intermolecular interaction and leads to a decrease in the density (Table 2).

Scheme 4. Arrangement of poly(para-aramid) and poly(meta-aramid) macromolecules in fiber crystals.
The reduced energy of hydrogen bonds in meta-aramid simplifies their cleavage during friction and intensifies tribochemical processes. This leads to a deeper friction trace and an increase in the area of friction contact, which, in turn, affords a dramatic increase in the friction coefficient. The structure of para-aramid, as can be seen from Scheme 4, is more rigid; the hydrogen bonds are extremely short (~ 4.7 Ǻ). It is more difficult to cleave them during friction than the hydrogen bonds in meta-aramid. The friction trace is localized on the surface.
Hence, in polyaramids, unlike aliphatic polyamides, the value of friction coefficient depends not only on the relative content (percentage) of amide groups in a macromolecule unit, but also on the structural factor caused by different arrangements of hydrogen bonds in a phenyl core (in the above case, para- or meta-disposition).
Unlike polyaramids, tribochemically active polymers that feature rather high friction coefficients, the organoplastic based on tribostable polyoxadiazole retains a low and stable value of the friction coefficient. It should be emphasized that the results of investigation did not reveal a correlation between the chemical structure of triboactive (polyaramids) and tribostable (POD) polymers and their tribological properties.
5. Self-organization in organoplastics based on PF polymer reinforced by a mixture of POD and cotton fibers
One of the most interesting tribochemical features of triboactive polymers is the propensity for self-organization of friction surfaces in a wide temperature range. This can be illustrated by self-organization of organoplastics based on PF polymer and a mixture of reinforcing fillers of cotton and polyoxadiazole (POD) fibers. The content of POD fibers in the compositions composed 100%, 70%, 50%, and 40% (Scheme 5). Curing of the binder was carried out at 160 °С for 15 min followed by thermal processing at 120 °С for 2 h.
|
Phenol formaldehyde |
|
|
Reinforcing cut |
|
|
Cotton cut fiber |
|
Scheme 5. Starting components of organoplastics. Resin : reinforcing filler ratio 40:60.
Figure 6 depicts the thermofriction dependences of the composites based on PF polymer.

Figure 6. Thermofriction dependences of the friction coefficients of organoplastics reinforced by a mixture (40%) of randomly distributed POD and cotton fibers during friction against steel. The compositions of the mixed fillers: 40% cotton and 60% POD fibers (1); 50% cotton and 50% POD fibers (2); 70% cotton and 30% POD fibers (3); 100% POD fibers (4); 100% cotton fibers (5). The test conditions: vertical friction machine, friction joint: butt ends of two hubs; P = 0.25 MPa; v = 0.5 m/s; rate of the temperature rise 10 °С/min.
The lowest value of the initial friction coefficient (0.15) was observed for the organoplastic bearing only POD fibers. As the temperature rose, the difference between the organoplastics bearing 100% POD fibers (curve 4) and 100% cotton fibers (curve 5) became more evident. The samples including both POD and cotton fibers featured almost the same values of initial f, which composed 0.20–0.22. At the elevated temperatures up to 135 °C, the values of their friction coefficients increased, but an insignificant difference between the values of f of the organoplastics with 100% POD fibers (curve 4) and a mixture of POD and cotton fibers (curves 1, 2, 3) was still detected. Interestingly, despite considerable differences in the filler compositions, the thermofriction tests of the organoplastics bearing mixed fillers revealed close friction and wear coefficients (Table 3).
Table 3. Effect of the content of POD fibers in a mixed filler on tribological properties
of the composites based on PF resin during thermofriction tests (P = 0.25 MPa, v = 0.5 m/s)
|
Content of POD |
Parameters at the end of thermofriction tests |
||
|
Friction coefficient |
Contact temperature, °С |
Wear, g |
|
|
100 |
0.35 |
135 |
0.039 |
|
70 |
0.45 |
137 |
0.043 |
|
50 |
0.44 |
133 |
0.050 |
|
40 |
0.38 |
133 |
0.045 |
This nontrivial result required further investigation of the structures of friction surfaces by XPS. The studies were concerned with the surfaces of samples obtained after the tests at 60 °С and 130 °С. According to the thermofriction dependences, the former temperature provided the samples with the lowest values of friction coefficients, whereas the latter temperature—afforded the highest coefficients. The results of XPS analysis of the organoplastics during friction tests under isothermal conditions at the temperatures of 60 °С and 130 °С are presented in Table 4.
Table 4. Elemental compositions of friction surfaces of the composites bearing different contents of POD fibers, at %.
The thicknesses of the analyzed surface layers were 50–100 Ǻ
|
Before friction |
|||||
|
Element |
Content of POD fibers relative to that of cotton fibers in the composition, % |
||||
|
100 |
70 |
50 |
40 |
0 |
|
|
С |
92.89 |
83.30 |
86.13 |
87.54 |
85.19 |
|
О |
7.11 |
15.65 |
13.87 |
12.46 |
14.82 |
|
N |
0.00 |
0.55 |
0.00 |
0.00 |
0.00 |
|
After friction at contact temperature of 60 °C |
|||||
|
Element |
100 |
70 |
50 |
40 |
0 |
|
С |
90.5 |
83.3 |
86.9 |
83.8 |
80.9 |
|
О |
7.4 |
15.7 |
13.1 |
15.2 |
19.1 |
|
N |
2.1 |
1.0 |
1.1 |
1.0 |
0.0 |
|
After friction at contact temperature of 130 °C |
|||||
|
Element |
100 |
70 |
50 |
40 |
0 |
|
С |
78 |
78.9 |
75 |
76.7 |
– |
|
О |
16.5 |
16.3 |
17.5 |
18.2 |
– |
|
N |
4.8 |
4.8 |
6.6 |
5 |
– |
First of all, we considered the atomic content of nitrogen in the XPS spectra, since this element is present only in POD fibers of the organoplastics explored. After friction at the elevated temperatures, nitrogen was found on the surfaces of all the samples, which indicates almost the same contents of POD fibers on their surfaces despite an essential difference in the contents of POD fibers in the initial samples. Thus, after friction at 60 °С, the contents of nitrogen for all the composites under investigation was about 1%, whereas at 130 °С the nitrogen contents reached 5–6%. This result is indicative of a dramatic change in the chemical composition of a composite surface at high friction temperature, which facilitates its enrichment with POD fibers. As a consequence, the difference in the contents of POD fibers on the resulting (more heat-resistant) friction surfaces for the composites with different initial fillers appeared to be insignificant.
The observed effect is likely to be connected with self-organization of the composites during friction. It includes rearrangement of the initial surface structure due to tribochemical processes of chemical flow in PF polymer [27], selective wear of less stable cotton fibers (Table 3), and, as a result, formation of the secondary oriented structure and more thermally stable surface (Fig. 7). The creation of such a surface owing to the mentioned processes is accompanied by its gradual enrichment with heat-resistant tribochemically stable POD fibers.
The reinforcement by POD fibers leads upon friction at 60 °C or 130 °С to the formation of discrete surfaces with supporting hard-to-deform POD fibers and PF overflows (Fig. 7) [28]. This is facilitated by the chemical flow of PF polymer in the contact zone, in the regions of the highest shear stresses. The friction surface (Fig. 7) reinforced by a mixture of fibers (70% POD + 30% cotton fibers) demonstrates PF overflows along with POD fibers rising above the surface, which are oriented in the sliding direction.
|
70% POD + 30% cotton fibers |
100% POD |
Hence, the revealed effect of self-organization of the friction process is defined by the use of active cross-linked PF polymer as a binder. It is well known that under temperature impact and strong shear strains, this polymer can change and then recover its shape owing to the cleavage and formation of chemical bonds in the course of chemical flow. An essential role in the creation of a secondary structure of cured PF polymers played not only by the covalent bonds, but also by the possibility of easy rearrangement of a network of hydrogen bonds during the formation of resite. Temperature and friction multiply amplify this process, and depending on the composition of polymer composite materials (PCM), it can proceed with variable intensity.
It should be emphasized that friction at the high temperature leads to the formation of a discrete surface in the polymer system under consideration. This provides an opportunity to reduce the wear of all compositions to close values despite different contents of temperature-sensitive cotton fiber (Table 4).
6. Conclusions
To prove a special character of friction of tribochemically active polymers, a range of linear and thermosetting polymers were analyzed both in the initial and reinforced forms. It was shown that the tribochemical activity in linear polymers is realized owing to the reaction of functional groups, which were remained upon processing or due to incompleteness of the curing process in thermosetting binders.
Particular interest to tribochemical activity of polyamides is connected with the effect of their amide groups on these processes. In the case of aliphatic polyamides, there is a clear relationship between the friction coefficient and the chemical structure of a polymer, which is expressed through a heterochain parameter. In aromatic heat-resistant polyaramids, the friction coefficient depends not only on the relative content (percentage) of amide groups in a macromolecule unit, but also on the structural factor, which is caused by different arrangement of hydrogen bonds in a phenol core; in the considered examples, it is para- or meta-disposition.
The effect of self-organization, revealed in organoplastics based on a tribochemically active phenol formaldehyde matrix, was found to be caused by several processes which lead to the formation of a new, more heat- and wear-resistant surface. This is associated with structure rearrangement of cured PF polymer under action of temperature and shear strains and selective wear of less stable cotton fibers, which leads to gradual enrichment of the surface with more thermally stable POD fibers. This gives rise not only to a heat-resistant oriented structure, but also to a discrete surface, which allows for realization of the relatively low wear despite the considerably increased friction point. Heat-resistant POD fibers serve on this surface as the elements that take up load, which essentially reduces the actual contact area.
Acknowledgements
The authors are grateful to Prof. O. A. Serenko for helpful discussion and recommendations.
References
1. A. P. Krasnov, V. A. Mit', O. V. Afonicheva, I. A. Rashkovan, M. E. Kazakov, Trenie Iznos, 2002, 23, 397–410.
2. M. O. W. Richardson, Wear, 1971, 17, 89–99. DOI: 10.1016/0043-1648(71)90021-4
3. A. P. Krasnov, A. V. Naumkin, V. N. Aderikha, D. I. Buyaev, I. O. Volkov, A. S. Yudin, M. V. Goroshkov, J. Frict. Wear, 2017, 38, 202–207. DOI: 10.3103/S1068366617030084
4. V. N. Aderikha, A. P. Krasnov, A. V. Naumkin, V. A. Shapovalov, Wear, 2017, 386–387, 63–71. DOI: 10.1016/j.wear.2017.04.022
5. A. P. Krasnov, A. V. Naumkin, V. N. Aderikha, A. S. Yudin, O. V. Afonicheva, K. I. Maslakov, A. S. Goloveshkin, N. D. Lenenko, I. S. Bushmarinov, S. S. Pesetsky, A. S. Golub', J. Frict. Wear, 2014, 35, 330–338. DOI: 10.3103/S1068366614040060
6. A. P. Krasnov, A. V. Naumkin, V. N. Aderikha, D. I. Buyaev, I. O. Volkov, A. S. Yudin, M. V. Goroshkov, J. Frict. Wear, 2017, 38, 202–207. DOI: 10.3103/S1068366617030084
7. A. P. Krasnov, I. A. Gribova, A. N. Chumaevskaya, J. Frict. Wear, 1997, 18, 110–126.
8. A. P. Krasnov, I. A. Gribova, V. N. Aderikha, Ya. V. Genin, J. Frict. Wear, 1999, 20, 99-105
9. A. P. Krasnov, L. S. Fedorova, V. A. Mit', L. Z. Rogovina, V. A. Vasil'ev, V. B. Riskin, J. Frict. Wear, 1996, 17, 32–37.
10. A. P. Krasnov, I. A. Gribova, O. V. Afonicheva, J. Frict. Wear, 1998, 19, 75–80.
11. A. S. Fialkov, Carbon-Graphite Materials, Energiya, Moscow, 1979 [in Russian].
12. A. P. Krasnov, L. S. Fedorova, O. V. Afonicheva, V. S. Papkov, M. I. Buzin, I. O. Volkov, J. Frict. Wear, 2001, 22, 66–70.
13. A. P. Krasnov, I. A. Rashkovan, O. V. Afonicheva, M. E. Kazakov, O. B. Kulachinskaya, O. V. Vinogradova, V. B. Bazhenova, V. A. Shirokov, Trenie Iznos, 2006, 27, 527–534.
14. V. V. Korshak, I. A. Gribova, A. P. Krasnov, I. K. Taratuta, M. G. Chudinov, B. N. Kuznetsov, Yu. S. Nekrasov, S. Sh. Trokhova, V. V. Anokhin, Vysokomol. Soedin., Ser. A., 1987, 29, 1699–1704.
15. V. V. Korshak, T. M. Frunze, Synthetic Heterochain Polyamides, AN SSSR, Moscow, 1962, p. 10 [in Russian].
16. A. P. Krasnov, O. V. Afonicheva, I. A. Rashkova, M. E. Kazakov, A. B. Popova, Proc. Int. Conf. "Polymer Composites 2000", Gomel, Belarus, 2000, pp. 21–24 [in Russian].
17. A. P. Krasnov, I. A. Rashkovan, M. E. Kazakov, O. V. Afonicheva, A. B. Popova, Vestn. Mashinostr., 2002, 25–28.
18. D. I. Buyaev, A. P. Krasnov, A. V. Naumkin, A. S. Yudin, O. V. Afonicheva, A. Yu. Golub', M. V. Goroshkov, M. I. Buzin, J. Frict. Wear, 2016, 37, 351–357. DOI: 10.3103/S106836661604005X
19. M. Jassal, S. Ghosh, Indian J. Fibre Text. Res., 2002, 27, 290–306.
20. E. G. Chatzi, J. L. Koenig, Polym.-Plast. Technol. Eng., 1987, 26, 229–270. DOI: 10.1080/03602558708071938
21. T. Huang, R. Lu, Y. Ma, P. Liu, T. Li, J. Macromol. Sci., Part B: Phys., 2012, 51, 109–124. DOI: 10.1080/00222348.2011.583195
22. H. Kakida, Y. Chatani, H. Tadokoro, J. Polym. Sci., Polym. Phys. Ed., 1976, 14, 427–435. DOI: 10.1002/pol.1976.180140305
23. V. V. Korshak, I. A. Gribova, A. P. Krasnov, G. V. Mamatsashvili, T. I. Dzhashiashvili, B. S. Lioznov, Trenie Iznos, 1984, 5, 265–271.
24. A. S. Yudin, D. I. Buyaev, O. V. Afonicheva, I. G. Goryacheva, A. P. Krasnov, J. Frict. Wear, 2013, 34, 245–252. DOI: 10.3103/S1068366613040120
25. A. A. Askadskii, V. I. Kondrashchenko, Computational Materials Science of Polymers, Nauchnyi Mir, Moscow, 1999 [in Russian].
26.Yu. A. Mikhailin, Thermally Stable Polymers and Polymer Materials, Professiya, St. Petersburg, 2006 [in Russian].
27. V. A. Kargin, G. L. Slonimskii, Short Sketches on Physical Chemistry of Polymers, Khimiya, Moscow, 1967 [in Russian].
28. M. O. Panova, A. P. Krasnov, A. V. Naumkin, L. F. Klabukova, N. D. Kagramanov, D. I. Buyaev, V. A. Solov'eva, J. Frict. Wear, 2018, 39, 462–468. DOI: 10.3103/S1068366618060119










