2024 Volume 7 Issues 1–3
|
INEOS OPEN, 2024, 7 (1–3), 18–20 Journal of Nesmeyanov Institute of Organoelement Compounds Download PDF |
|
Poly(trifluoroethylacrylatemethylsiloxane) and Polydecylmethylsiloxane Copolymer:
A New Polymer for Membrane Applications
Topchiev Institute of Petrochemical Synthesis, Russian Academy of Sciences, Leninskii pr. 29, Moscow, 119991 Russia
Corresponding author: E. A. Grushevenko, e-mail: evgrushevenko@ips.ac.ru
Received 10 May 2024; accepted 30 May 2024
Abstract
A series of poly(trifluoroethylacrylatemethylsiloxane) (F3) and polydecylmethylsiloxane (C10) copolymers featuring different ratios of the side groups were synthesized for the first time. A significant change in the sorption of the components of the ABE-fermentation mixture was demonstrated when the ratio of C10 and F3 in the membrane material changed. It was established that the copolymer with the C10/F3 ratio of 1:1 exhibits the maximum value of n-butanol sorption. At the same time, the total flow through the membrane doubles in comparison with C10 and it is possible to stabilize the flow by 15% after prolonged contact with the real ABE mixture.
Key words: poly(trifluoroethylacrylatemethylsiloxane), polydecylmethylsiloxane, membrane, ABE fermentation.
Introduction
The growing emphasis on the reduction of carbon footprints and transition to sustainable energy sources has highlighted the importance of biofuels in the fuel industry [1]. One key technology for producing biobutanol is Acetone–Butanol-Ethanol (ABE) fermentation using Clostridium bacteria. However, traditional ABE fermentation is limited by the low biobutanol concentration in the broth, making distillation separation challenging [2]. To address this issue, methods like hydrophobic pervaporation have been proposed for continuous biobutanol separation from the fermentation mixture. Pervaporation offers energy efficiency with lower energy consumption compared to distillation and does not harm microorganisms while operating at low temperatures [3].
Most commercial pervaporation membranes are silicone rubber-based polymers, mainly involving polydimethylsiloxane (PDMS) due to its performance and cost [4]. However, PDMS-based membranes lack sufficient selectivity for butanol. Hence, the development of polysiloxane-based membranes with better selectivity towards butanol is important. Another challenge in pervaporation is membrane fouling. The introduction of fluorine-containing fragments into PDMS significantly reduces membrane fouling by components of the fermentation mixture and increases the stability of the transport properties of the membrane during the pervaporation release of butanol from the ABE-fermentation mixture [5].
The use of the hydrosilylation reaction [6] and a one-step approach to the preparation of membranes based on polyorganosiloxanes [7] can significantly expand the chemistry of polymers used for the pervaporative separation of ABE mixtures. In this work, a series of copolymers of poly(trifluoroethylacrylatemethylsiloxane) (F3) and polydecylmethylsiloxane (C10) with different ratios of the side groups (from 0 to 100%) was obtained for the first time.
Results and discussion
The hydrosilylation of polymethylhydrosiloxane (PMHS) by 1-decene and trifluoroethylacrylate were assessed by analyzing the 1H NMR spectra of the reaction mixtures of fluorinated polymethylsiloxanes (see Fig. S1 in the Electronic supplementary information (ESI)). It was shown that fluoroalkyl acrylate substituents add across the C=C bond against Markovnikov's rule over the entire range of the copolymer ratios, which corresponds to the generally accepted concept of the hydrosilylation reaction occurring in the presence of Karstedt's catalyst [6]. The conversion of CH2=CH bonds of F3 into side agents decreases with an increasing in their amount: 91% for C10/F3 ratio of 0:100, 94% for 50:50, and 96% for 95:5.
The dependence of the sorption of the components of the ABE mixture on the content of F3 in the copolymer is shown in Fig. 1. Unlike acetone and ethanol, the sorption of which linearly depends on the content of F3, for n-butanol a maximum is observed at the C10/F3 ratio of 1:1. This may be due to the specific supramolecular structure of the polymer. This composition is most interesting for use in the pervaporative separation of the ABE mixture.
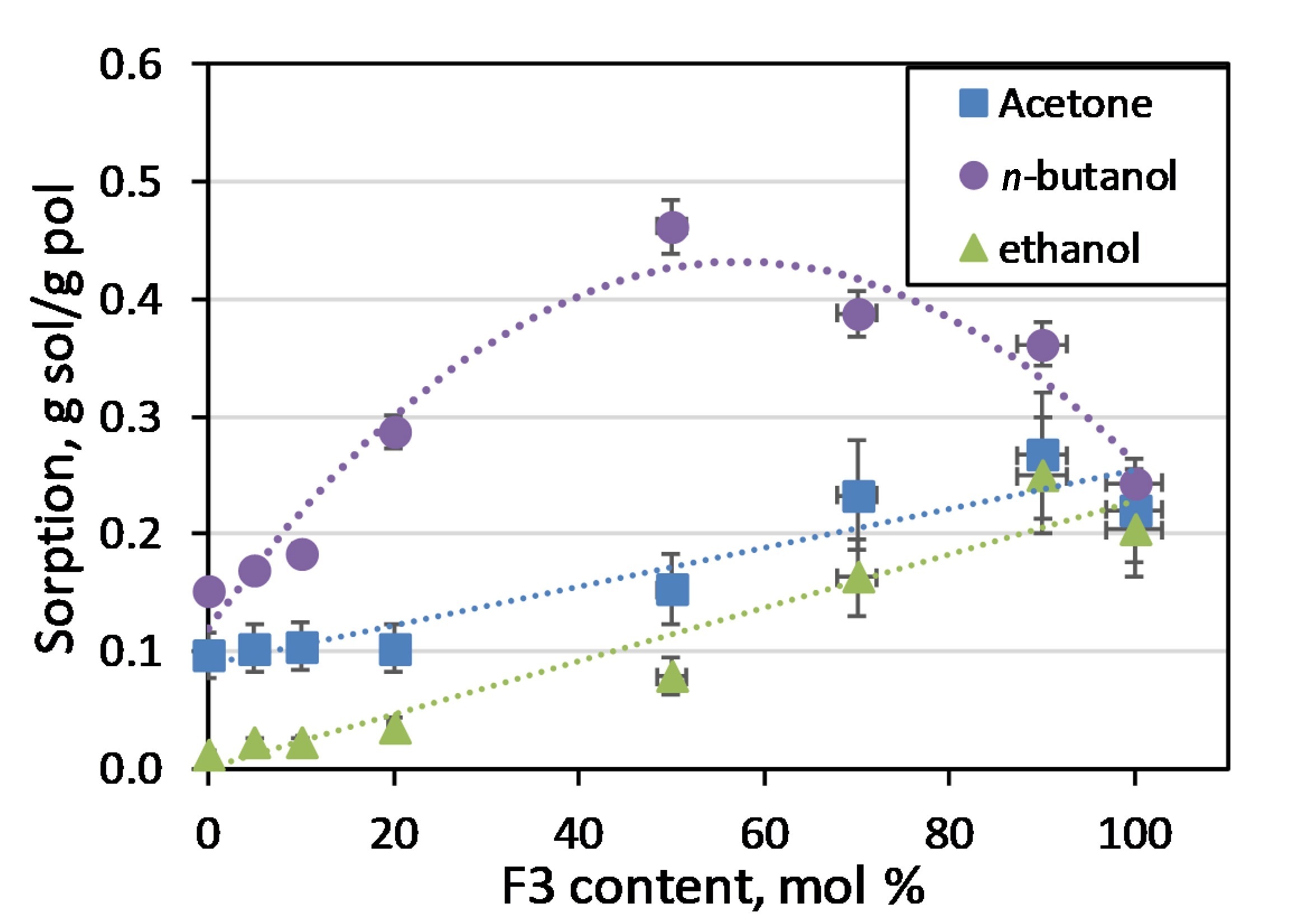
Figure 1. Dependence of the sorption of the ABE mixture components on the F3 content in the copolymer.
A comparison of the stability of the pervaporation properties of C10 and C10–F3 before and after contact with the fermentation mixture demonstrates that the introduction of F3 fragments makes it possible to increase the stability of the permeate flow by 15%. It is worth noting that the total flux for C10–F3 was 49 g/(m2h), which is almost twice as high as that for C10. The n-butanol/water separation factor remained at the same level: 40 ± 2.
Experimental section
Copolymer synthesis
The copolymers of F3 and C10 were obtained by the hydrosilylation reaction according to the one-step in situ methodology previously suggested for polyalkylmethylsiloxanes (Scheme 1) [7]. PMHS was mixed with a 15 wt % solution of trifluoroethylacrylate or/and 1-decene in toluene and Karstedt's catalyst. The ratio of 1-decene to trifluoroethylacrylate varied from 0:100 to 100:0. The resulting mixture was stirred at 60 °C for 2 h. To cross-link the polymer, a 10 wt % solution of PDMS in toluene was added to the reaction mixture. The stirring was continued at 60 °C for 1 h. At the final step, a 3 wt % solution of PMHS was added up to the PMHS/PDMS molar ratio of 0.16. The thickness of the resulting film was 70 ± 5 μm.
Scheme 1. Synthesis of the copolymer.
Methods
The composition of the reaction mixture after the interaction of PMHS and side substituents was analyzed using NMR spectroscopy. The high-resolution 1H NMR spectra were registered on a Bruker AVANCE III HD 400 NMR spectrometer in CDCl3 solutions using the standard procedures.
To evaluate the sorption interaction between n-butanol, acetone, and ethanol with the polymers, the gravimetric method was used to determine the equilibrium sorption, as described in Ref. [8].
A polymer sample with a diameter of 2 cm and a thickness of approximately 150 μm was immersed into the test liquid and held at 30 °C for 48 h, until a constant mass was reached. After removing the sample from the liquid, any excess moisture on the surface was removed, and the sample was weighed using a Sartorius Analytic A120S balance. This procedure was repeated three times for each polymer/liquid combination. The equilibrium sorption value was determined using the following formula:

where m1 and m2 are the masses of the polymer before and after sorption, g.
The transport (flux) and separation factor of the membranes were investigated by the pervaporative separation of the model ABE-fermentation mixture containing 0.76 wt % of acetone, 1.6 wt % of BuOH, and 0.3 wt % of EtOH in water at 30, 40, and 50 °C. The separation process was performed in a vacuum pervaporation setup as described elsewhere [9]. The membrane resistance to clogging was assessed based on a difference in the membrane permeability before and after prolonged contact with the real ABE broth.
Conclusions
Hence, a series of C10–F3 copolymers were synthesized for the first time. The hydrosilylation degree of PMHS was demonstrated to be more than 90% at the stage of the polymer modification. It was found that, when the ratio of side groups F3 and C10 changes, the sorption capacity for n-butanol passes through a maximum at an equal theoretical ratio of the groups. The resulting membrane materials demonstrated good transport properties and relative fouling stability, which indicates their potential in the ABE-broth pervaporative separation.
Acknowledgements
This work was supported by the Russian Science Foundation (project no. 22-79-10332).
Electronic supplementary information
Electronic supplementary information (ESI) available online: the NMR spectra and molecular-weight characteristics of the compounds explored. For ESI, see DOI: 10.32931/io2409a.
References
- B. Mridha, G.V. Ramana, S. Pareek, B. Sarkar, Fuel, 2023, 336, 126896. DOI: 10.1016/j.fuel.2022.126896
- Z. Lin, W. Cong, J. Zhang, Fermentation, 2023, 9, 847. DOI: 10.3390/fermentation9090847
- A. Oudshoorn, L. A. M. van der Wielen, A. J. J. Straathof, Ind. Eng. Chem. Res., 2009, 48, 7325–7336. DOI: 10.1021/ie900537w
- Z. Si, H. Wu, P. Qin, B. Van der Bruggen, Sep. Purif. Technol., 2022, 298, 121612. DOI: 10.1016/j.seppur.2022.121612
- H. Zhu, X. Li, Y. Pan, G. Liu, H. Wu, M. Jiang, W. Jin, J. Membr. Sci., 2020, 609, 118225. DOI: 10.1016/j.memsci.2020.118225
- Hydrosilylation: A Comprehensive Review on Recent Advances, B. Marciniec (Ed.), Springer, Dordrecht, 2009. DOI: 10.1007/978-1-4020-8172-9
- I. L. Borisov, E. A. Grushevenko, T. S. Anokhina, D. S. Bakhtin, I. S. Levin, G. N. Bondarenko, V. V. Volkov, A. V. Volkov, Mater. Today Chem., 2021, 22, 100598. DOI: 10.1016/j.mtchem.2021.100598
- S. Darvishmanesh, J. Degrève, B. Van der Bruggen, Chem. Eng. Sci., 2009, 64, 3914–3927. DOI: 10.1016/j.ces.2009.05.032
- E. A. Grushevenko, I. A. Podtynnikov, I. L. Borisov, Russ. J. Appl. Chem., 2019, 92, 1593–1601. DOI: 10.1134/S1070427219110168





