2024 Volume 7 Issues 1–3
|
INEOS OPEN, 2024, 7 (1–3), 9–10 Journal of Nesmeyanov Institute of Organoelement Compounds Download PDF |
|
Preparation of Allyl-Containing PDMS Telehelics by the
Anionic Ring-Opening Polymerization
S. A. Milenin,a,b,c and A. M. Muzafarov a
a Enikolopov Institute of Synthetic Polymeric Materials, Russian Academy of Sciences, ul. Profsoyuznaya 70, Moscow, 117393 Russia
b Research Laboratory of New Silicone Materials and Technologies, Tula State Lev Tolstoy Pedagogical University, pr. Lenina 125, Tula, Tula Oblast, 300026 Russia
c Center of National Technological Initiative, Bauman Moscow State Technical University, 2-ya Baumanskaya ul. 5, Moscow, 105005 Russia
Corresponding author: K. A. Bezlepkina, e-mail: bezlepkina_ka@ispm.ru
Received 10 May 2024; accepted 1 June 2024
Abstract
The synthesis of allyl-containing PDMS telechelics from octamethylcyclotetrasiloxane and 1,3-diallyl-1,1,3,3-tetramethyldisiloxane as a stopper by the anionic ring-opening of a siloxane ring is presented. A series of the polymers with the molecular weights from 1000 to 12000 were obtained and characterized by gel permeation chromatography and 1H NMR spectroscopy. The molecular weights of the resulting polymers correspond to the given ones.
Key words: polysiloxanes, catalytic rearrangement, ring-opening polymerization, polydiorganosiloxane telechelics, allylsiloxanes.
Introduction
Polydimethylsiloxane telechelics (PDMSs) with different organic shells amount to the first products with well-defined structures in this class of high-molecular compounds, used as reagents for the targeted molecular design of siloxane polymers and materials. The method of a siloxane ring opening is one of the first reactions in the polymer chemistry of silicones [1–3].
Herein, the synthesis of allyl-containing PDMS telechelics by the anionic opening of a siloxane ring is presented [4, 5]. The resulting polymers are promising for the production of materials by the hydrosilylation reaction [6, 7]. The advantage of this process is the simplicity of performance and isolation of the target products, which includes the catalyst decomposition at elevated temperatures and evaporation of the volatile products [8]. Earlier it was used for the preparation of only one allyl-containing PDMS [9], but the details of the synthesis were not described in the paper.
Results and discussion
In this work, we demonstrated the synthesis of allyl-containing PDMS telechelics by the mechanism of the anionic opening of a siloxane ring of octamethylcyclotetrasiloxane (D4) in the presence of 1,3-diallyl-1,1,3,3-tetramethyldisiloxane as a chain terminator and tetramethylammonium hydroxide (TMAH) as a catalyst (Scheme 1).
Scheme 1. Synthesis of the allyl-containing PDMSs.
The process was carried out at 100 °C for 5 h until the formation of the maximum content of high-molecular products, which was monitored by gel permeation chromatography (GPC) (see the Electronic supplementary information (ESI)). The calculated and obtained molecular weights of the resulting polymers are presented in Table 1.
Table 1. Molecular-weight characteristics of the resulting polymers
|
Polymer
|
Calc. MW
|
MW (NMR)
|
Mn
|
Mw
|
Mw/Mn
|
|
1
|
1000
|
1350
|
1480
|
2210
|
1.50
|
|
2
|
3000
|
3430
|
4100
|
7100
|
1.71
|
|
3
|
5000
|
4770
|
6100
|
10000
|
1.60
|
|
4
|
8000
|
7140
|
9200
|
16100
|
1.75
|
|
5
|
12000
|
10480
|
12300
|
21600
|
1.76
|
The formation of the polymers with terminal allylsilyl groups was confirmed by 1H NMR spectroscopy (Fig. 1). The presence of the allyl proton signals in the spectra indicated their presence in the polymer. A coincidence of the ratio of the integral intensities of the allyl and methyl proton signals with the theoretical one demonstrates the possibilities of this method for the synthesis of a polymer with the given molecular weight.
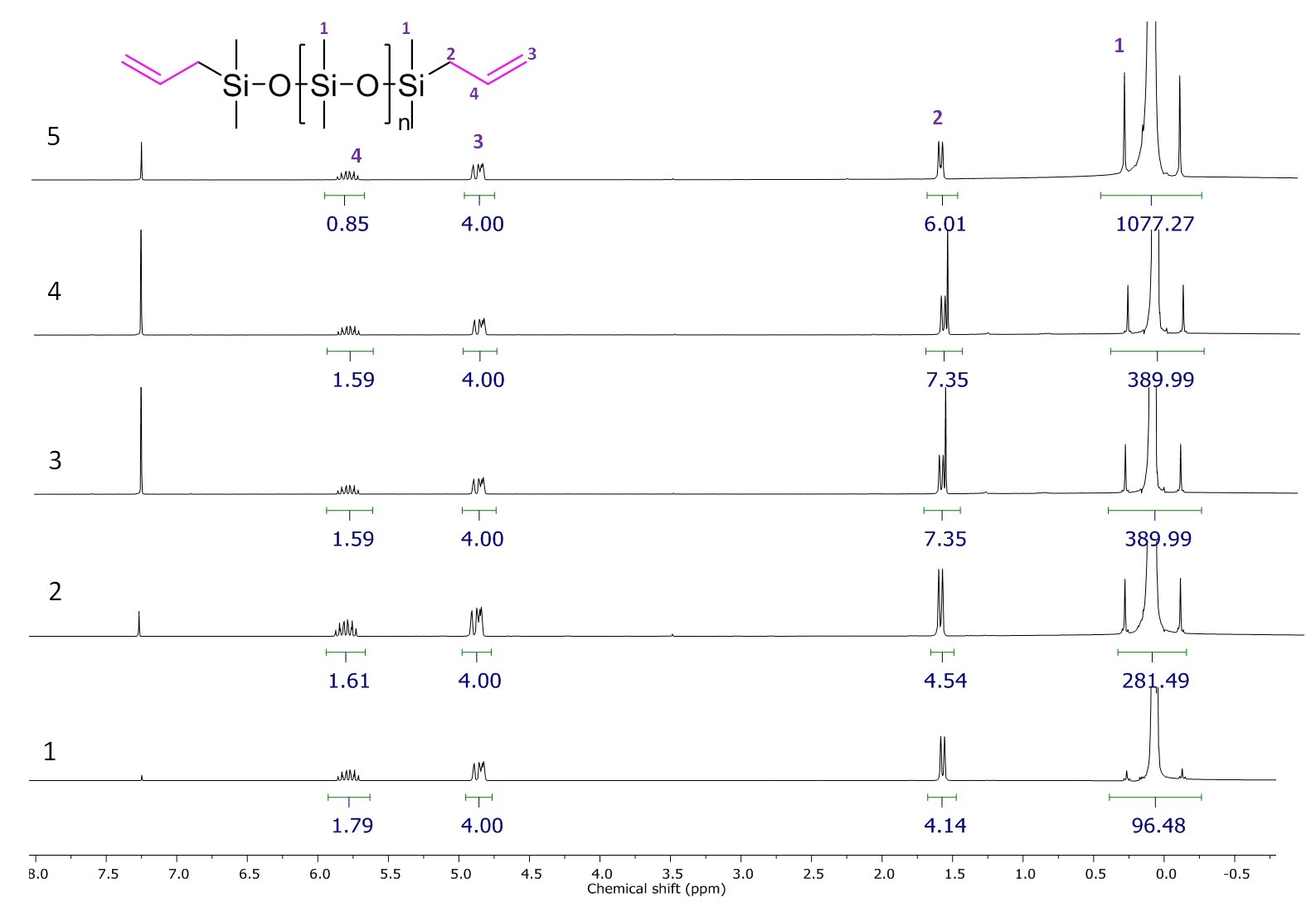
Figure 1. 1H NMR spectra of the resulting polymers.
The yields of the siloxane products ranged from 90 to 98%, while the content of the high-molecular polymer according to the GPC data was about 87%.
Experimental section
General remarks
All the reagents were used as received. Octamethylcyclotetrasiloxane (D4) was purchased from ABCR. Tetramethylammonium hydroxide (TMAH) was purchased from Acros (Belgium).
Analysis and general methodology
The 1H NMR spectra were recorded on a Bruker Avance AV-300 spectrometer (300 MHz for 1H).
The GPC analysis was performed on a Shimadzu LC-10A series chromatograph (Japan) equipped with an RID-10A refractometer and SPDM10A diode matrix detectors. The analytical separation was performed using a 7.8 mm × 300 mm Phenomenex column (USA) filled with the Phenogel sorbent with the pore sizes of 15–500 Å.
Syntheses
General procedure for the synthesis of the allyl-PDMSn-allyl telechelics (1–5). A stirred mixture of 1,3-diallyl-1,1,3,3-tetramethyldisiloxane, octamethylcyclotetrasiloxane, and tetramethylammonium hydroxide (TMAH) was heated at 100 °C for 5 h. The conversion was monitored using GPC based on the depletion of the reaction mixture with D4. The catalyst was removed by the thermal decomposition at 150 °C for 1 h. The following evaporation of the volatiles (p = 0.5 bar, 100 °C) afforded the target product.
Conclusions
Based on our investigations, we proposed a simple method for obtaining PDMS telechelics with the allyl functional groups by the anionic opening of a siloxane ring. The resulting compounds are promising for the production of materials by the hydrosilylation reaction.
Acknowledgements
This work was partially supported by the Russian Science Foundation (project no. 21-73-30030) and the Government of the Tula Region (Decree No. 899 of December 30, 2021) within the framework of Agreement No. 899. 11, September 7, 2022.
The molecular-weight distribution and NMR spectroscopic studies were performed with financial support from the Ministry of Science and Higher Education of the Russian Federation (FFSM-2021-0004) using the equipment of the Collaborative Access Center "Center for Polymer Research" of ISPM RAS.
Electronic supplementary information
Electronic supplementary information (ESI) available online: the detailed synthetic procedures, the GPC curves, and the NMR spectra of the resulting compounds. For ESI, see DOI: 10.32931/io2405a.
References
- Silicon-Containing Polymers: The Science and Technology of Their Synthesis and Applications, R. G. Jones, W. Ando, J. Chojnowski (Eds.), Springer, Dordrecht, 2000. DOI: 10.1007/978-94-011-3939-7
- K. A. Bezlepkina, S. A. Milenin, N. G. Vasilenko, A. M. Muzafarov, Polymers, 2022, 14, 2408. DOI: 10.3390/polym14122408
- S. A. Milenin, F. V. Drozdov, K. A. Bezlepkina, V. Yu. Majorov, A. M. Muzafarov, Macromolecules, 2021, 54, 2921–2935. DOI: 10.1021/acs.macromol.0c02790
- F. B. Madsen, I. Javakhishvili, R. E. Jensen, A. E. Daugaard, S. Hvilsted, A. L. Skov, Polym. Chem., 2014, 5, 7054–7061. DOI: 10.1039/C4PY00919C
- A. Dietrich, E. Mejía, Eur. Polym. J., 2020, 122, 109377. DOI: 10.1016/j.eurpolymj.2019.109377
- B. Marciniec, H. Maciejewski, W. Duczmal, R. Fiedorow, D. Kityński, Appl. Organomet. Chem., 2003, 17, 127–134. DOI: 10.1002/aoc.402
- D. T. Hurd, G. F. Roedel, Ind. Eng. Chem., 1948, 40, 2078–2081. DOI: 10.1021/ie50467a015
- K. A. Bezlepkina, S. N. Ardabevskaia, K. S. Klokova, A. I. Ryzhkov, D. A. Migulin, F. V. Drozdov, G. V. Cherkaev, A. M. Muzafarov, S. A. Milenin, ACS Appl. Polym. Mater., 2022, 4, 6770–6783. DOI: 10.1021/acsapm.2c01265
- US Patent WO2023183682A1, 2023.





