2024 Volume 7 Issues 1–3
|
INEOS OPEN, 2024, 7 (1–3), 1–2 Journal of Nesmeyanov Institute of Organoelement Compounds |
|
Solubility Study of a Ladder-Like Polyphenylsilsesquioxane for Membrane Application
A. A. Anisimov,b,c O. I. Shchegolikhina,a,b A. V. Volkov,a and A. M. Muzafarov b,d
a Topchiev Institute of Petrochemical Synthesis, Russian Academy of Sciences, Leninskii pr. 29, Moscow, 119991 Russia
b Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, str. 1, Moscow, 119334 Russia
c Faculty of Electronics, Photonics and Molecular Physics, Moscow Institute of Physics and Technology (National Research University), Institutskiy per. 9, Dolgoprudny, Moscow Oblast, 141700 Russia
d Enikolopov Institute of Synthetic Polymeric Materials, Russian Academy of Sciences, ul. Profsoyuznaya 70, Moscow, 117393 Russia
Corresponding author: T. S. Anokhina, e-mail: tsanokhina@ips.ac.ru
Received 10 May 2024; accepted 24 June 2024
Abstract
In this work, the solubility of a high-molecular-weight homopolymer, a ladder polyphenylsilsesquioxane, was studied in different classes of solvents, including alcohols, aprotic solvents, ketones, alkanes, and aromatic hydrocarbons. The Hansen parameters and the parameter of long-range interaction between the polymer and the solvent were analyzed. The insolubility of L-PPSQ in a wide range of solvents allows for expecting that the properties of a membrane on its basis would be stable during operation in these media. At the same time, aprotic solvents are well suited for the preparation of membranes based on L-PPSQ by the solution phase inversion method.
Key words: polyphenylsilsesquioxanes, solubility, Hansen parameters, membranes.
Introduction
Membrane separation processes hold great promise for CO2 separation and separation of aromatic/aliphatic hydrocarbons. Membrane technologies can significantly reduce separation costs owing to compactness, modularity, greater separation efficiency, and reduced energy costs compared to the traditional processes. High permeability, selectivity, and stability at elevated temperatures are the key factors in choosing membrane materials and developing membranes based on them.
A special place among polymers is occupied by ladder polyphenylsilsesquioxanes (L-PPSQs) that represent glassy polymers with high thermal stability (decomposition onset temperature 495 °C) even in the presence of water vapor. The recently developed original method for the synthesis of L-PPSQ [1] afforded the polymer with a high molecular weight (1000000 g/mol) and, as a consequence, good mechanical (tensile strength 39 MPa and elongation at break 9%) and film-forming properties. The high thermal stability of L-PPSQ (Tg > Td > 490 °C) (even in the presence of water vapor) and high mechanical characteristics make this polymer unique among the existing membrane materials.
Despite the widespread use of L-PPSQ block copolymers in membrane science and technology, the solubility, mechanical and transport properties of L-PPSQ homopolymers have been largely unexplored. There is only one report from the early 1990s that deals with the investigation of the gas permeability of L-PPSQ [2]; however, the structure of the polymer practically was not explored. Herein, the solubility of the homopolymer in a number of organic solvents was investigated. This is necessary for choosing solvents in the production of membranes as well as their application.
Results and discussion
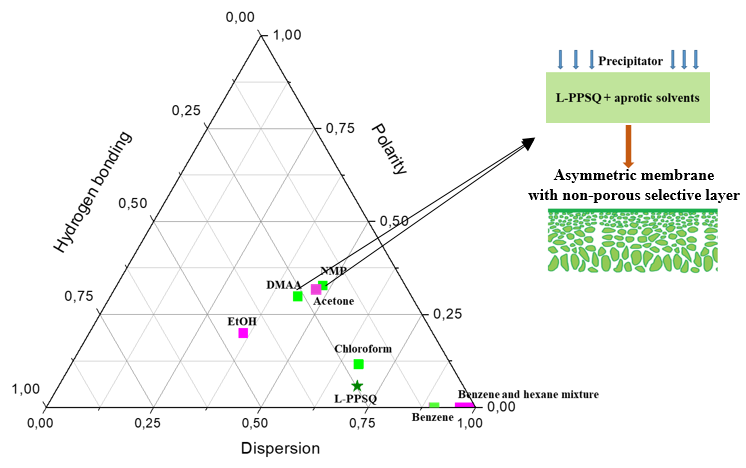
At the first stage, the solubility of L-PPSQ in seven organic solvents of various natures (chloroform, benzene, N-methylpyrrolidone (NMP), dimethylacetamide, hexane, acetone, and ethanol) and two organic mixtures (benzene/ethanol of different compositions) was studied and their Hansen solubility parameters were analyzed (Fig. 1) [3]. L-PPSQ under investigation had the following molecular-weight characteristics: Mw = 1057 kg/mol, Mp = 1731 kg/mol, Mn = 344 kg/mol. The solubility parameters (δ) were calculated taking into account the group contributions using equations (1–3):
 |
(1) |
| (2) | |
| (3) |
where Ecoh is the cohesion energy, Vm is the molar volume of the i-th functional group of the molecule, Ed is the dispersion component of energy, Ep is the polar component of energy, Eh is the energy of hydrogen bonds, δd is the contribution of dispersion interaction, δp is the contribution of polar interaction, and δh is the contribution of hydrogen bonds.

Figure 1. Comparison of the Hansen solubility parameters for L-PPSQ and its solvents.
In Fig. 1, the solvents (chloroform, benzene and polar aprotic amide solvents NMP and DMA) for L-PPSQ are highlighted in green. Solvents (hexane, acetone, ethanol, and mixtures of hexane and benzene) in which L-PPSQ is insoluble are highlighted in purple. This behavior is characteristic of PPSQ. Different research groups reported the solubility of materials of this class in benzene, chloroform and aprotic solvents (THF, DMF) and their insolubility in ketones, alcohols, ethers, and saturated cyclic and aliphatic hydrocarbons [4]. The insolubility of L-PPSQ in a wide range of solvents allows for expecting that the properties of a membrane on its basis would be stable during the operation in these media. Apparently, a definite advantage of L-PPSQ is that it is soluble in solvents with various viscosities, which ensures the development of membranes with good performance characteristics on its basis.
One of the ways to predict the sorption affinity of a polymeric material and a solvent is to determine the parameter of their long-range interaction. To estimate such interaction, a difference in the solubility parameters of the polymer and the solvent is used, taking into account the contributions of polar and dispersion interactions, as well as hydrogen bonds [5].
The smaller the difference between the solubility parameters of a polymer and a solvent, the higher the affinity between them. Therefore, one should expect high values of solvent sorption in the polymeric material [6]. The affinity of the i-th solvent (s) to the polymeric material (p) can be estimated by the long-range parameter
 |
(4) |
Based on the resulting values of the long-range interaction parameters (see Table 1), it can be concluded that benzene and chloroform are the best solvents for L-PPSQ among the studied examples. The interaction of L-PPSQ with NMP and DMA appears to occur at a boundary between good solvents in terms of the long-range parameter value (NMP: 10.6 MPa1/2, DMA: 10.5 MPa1/2). In a benzene/n-hexane 30/70 wt % mixture, L-PPSQ swells strongly but does not dissolve. L-PPSQ is insoluble in neat hexane, acetone, and ethanol. Moreover, this polymer does not swell at all in ethanol and hexane, while its behavior in acetone requires further study. Hence, it can be assumed that the long-range interaction parameter above 10.5–10.6 delineates the region of poor solvents.
Table 1. Estimation of the long-range interaction between the polymer and the solvents of various nature
|
Compound
|
|
|
L-PPSQ
|
–
|
|
Chloroform
|
3.11
|
|
Benzene
|
6.0
|
|
NMP
|
10.6
|
|
DMA
|
10.5
|
|
Hexane
|
9.3
|
|
Acetone
|
9.7
|
|
Ethanol
|
14.5
|
|
Benzene/hexane 10/90
|
11.3
|
|
Benzene/hexane 30/70
|
10.5
|
Conclusions
The solubility of L-PPSQ in various organic solvents was investigated and their Hansen solubility parameters were analyzed. Based on the values of the long-range interaction parameters
Acknowledgements
This work was supported by the Russian Science Foundation (project no. 23-79-10256).
References
- T. O. Ershova, A. A. Anisimov, M. N. Temnikov, M. A. Novikov, M. I. Buzin, G. G. Nikiforova, Yu. S. Duyzhikova, I. E. Ushakov, O. I. Shchegolikhina, A. M. Muzafarov, Polymers, 2021, 13, 4452. DOI: 10.3390/polym13244452
- Y. Mi, S. A. Stern, J. Polym. Sci., Part B: Polym. Phys., 1991, 29, 389–393. DOI: 10.1002/polb.1991.090290401
- C. M. Hansen, Hansen Solubility Parameters. A User's Handbook, 2nd ed., CRC Press, Boca Raton, 2007.
- R. H. Baney, M. Itoh, A. Sakakibara, T. Suzuki, Chem. Rev., 1995, 95, 1409–1430. DOI: 10.1021/cr00037a012
- S. Darvishmanesh, J. Degrève, B. Van der Bruggen, Phys. Chem. Chem. Phys., 2010, 12, 13333–13342. DOI: 10.1039/c0cp00230e
- E. S. Tarleton, J. P. Robinson, S. J. Smith, J. J. W. Na, J. Membr. Sci., 2005, 261, 129–135. DOI: 10.1016/j.memsci.2005.02.037





