2023 Volume 6 Issue 5 (Published 20 August 2024)
|
INEOS OPEN, 2023, 6 (5), 150–155 Journal of Nesmeyanov Institute of Organoelement Compounds |
|
Jumping Exothermic Effect in Polymer Gels
Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, str. 1, Moscow, 119334 Russia
Corresponding author: V. G. Vasiliev, e-mail: viktor@ineos.ac.ru
Received 2 February 2024; accepted 26 March 2024
Abstract
The specific reaction of polymer gels to the effect of an alternating temperature field is explored that is manifested in an exothermic effect, the position of which on the temperature scale depends on the thermal prehistory of the gel. It is found that the described exothermic effect is observed in polymer gels of any nature, and for its manifestation it is necessary to have a spatial network structure in which both free and bound solvent molecules are present.
Key words: exothermic effect, polymer gels, spatial network structure, free solvent, thermal prehistory.
Introduction
Polymer gels represent a binary polymer–solvent system in which there is a spatial network between macromolecules or their associates. The presence of a yield point and a large amount of solvent allows for considering gels as an intermediate substance between solutions and block polymers. These systems retain their shape under the influence of their own weight, and this is their major difference from polymer solutions. All existing polymer gels are divided into two classes: irreversible (chemical) and reversible (physical) gels [1–3].
The spatial network structure formed by covalent bonds is thermally irreversible. The cross-linking units of such a network are quite resistant to temperature or external mechanical impact. In such gels, there is no melting of the network, and the development of irreversible deformation is possible only through the mechanism of chemical flow, which is associated with the mechanical destruction of the spatial network.
A common and basic property of any physical gels is that the network cross-links are reversible; they are formed and decompose upon changing of temperature, pressure, or solvent composition (addition of a precipitant, pH variation, introduction of various additives, etc.) [4–6]. The existence of different types of spatial network structures leads to a number of differences in the gel response to the external influences of mechanical or temperature fields. In this work, the specific reaction of polymer gels to the effect of an alternating temperature field is considered that is manifested in an exothermic effect. Earlier a low-temperature exothermic effect was observed in aqueous gels of agarose [7, 8], which was explained as a manifestation of syneresis, while the exothermic effect in aqueous gels of ionomers based on sulfonated polyphenylquinoxalines [9] was associated with the exchange processes between the polymer and the solvent. Both of these explanations are rather declarative in nature and do not answer the question about the reasons of the observed phenomenon. In other polymer gels, a similar exothermic effect has not yet been described in the literature. The goal of this study was to ascertain the reasons and specifics of the manifestation of the detected exothermic effect.
The work was concerned with the most typical representatives of polymer gels featuring both a reversible (aqueous agar-agar gels) and irreversible (aqueous polyacrylamide (PAA) and polydimethylsiloxane (PDMS) gels in organic solvents) network structure.
Results and discussion
Research objects
The agar-agar gels were obtained according to the standard procedure [10] using Agar NF, Grade 9002-18-0 (MP Biochemicals). Agar-agar was dissolved at 90 °C. The resulting solution was cooled to room temperature. The gel formed upon cooling was kept at room temperature for 24 h and then placed in a calorimeter measuring cell and cooled to 5 °C.
The PAA gels were prepared according to the standard procedure [10] by polymerization of a 20 wt % aqueous solution of acrylamide (CH2=CH–CONH2) in the presence of a cross-linking agent N,N'-methylenebisacrylamide (MBA), (CH2=CH–CONH)2CH2, and an initiating system consisting of ammonium persulfate and tetramethylethylenediamine. The cross-linking extent was varied by the amount of MBA introduced into the reaction. The resulting network polymers were swollen in distilled water to constant mass for 7 days, replacing the solvent every day to remove a sol fraction. Then the swollen polymer was dried to constant mass and swollen again in water to an equilibrium state. Knowing the mass of the dry polymer, the mass fraction of the polymer in the equilibrium swollen gel (w2) was determined. The synthesis of PDMS networks with different molar masses of cross-linking units was carried out by reacting α,ω-dihydroxypolydimethylsiloxane (Aldrich) featuring Mn = 550 and 18000 with tetraethoxysilane in the presence of tin diethyl dicaprylate used as a catalyst [11]. The resulting cross-linked polymers were then extracted with heptane in a Soxhlet apparatus for 7 days to remove a sol fraction and the catalyst residues. The swollen networks were dried to constant mass and swollen again in the solvent to an equilibrium state.
The choice of the research objects was stipulated by the widespread use of these gels in various fields of industry; therefore, their properties are well studied and do not raise additional questions beyond the scope of this study.
Figure 1 depicts the DSC curves of the agar-agar and PAA aqueous gels, as well as the PDMS gel in heptane.
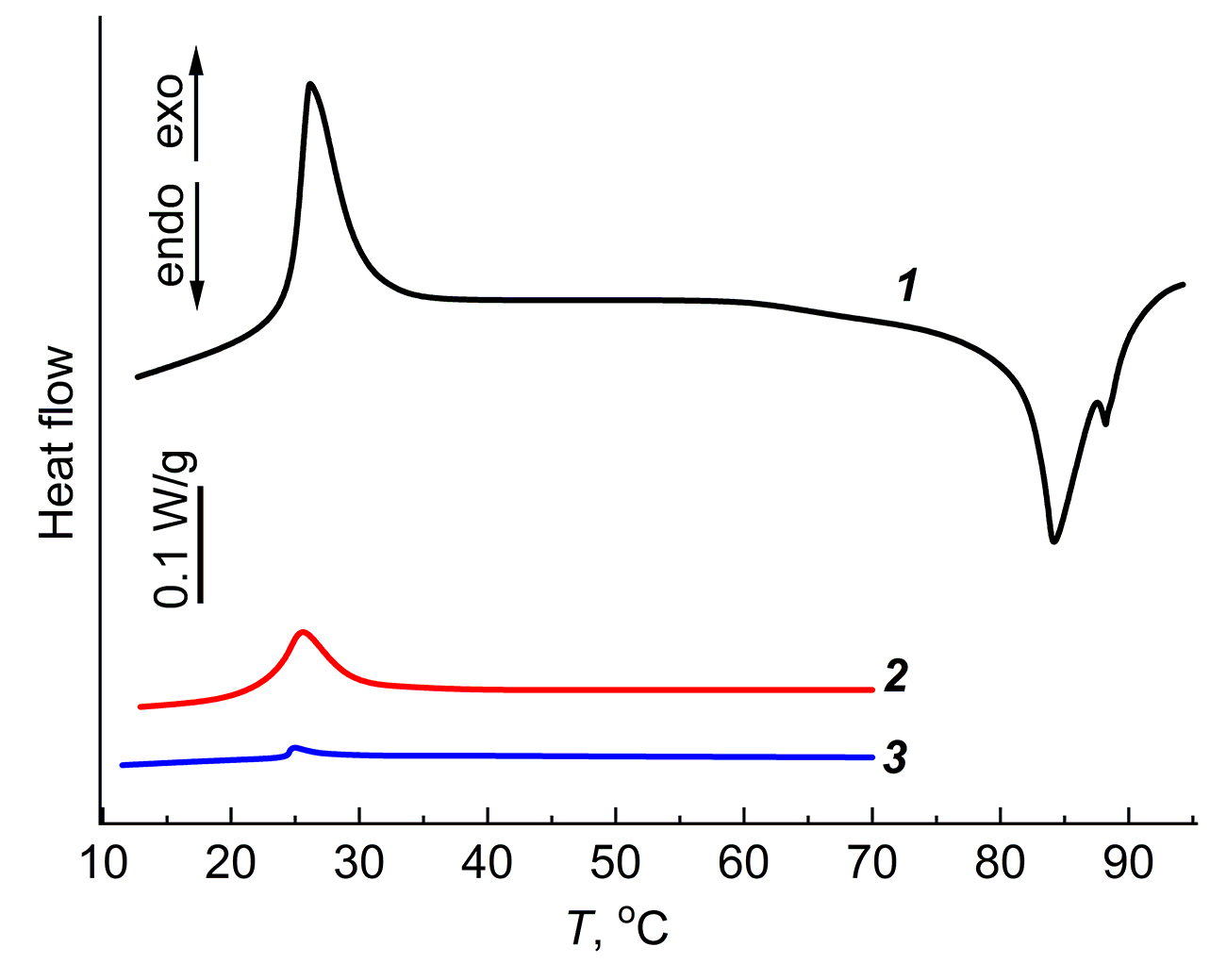
Figure 1. DSC curves of the aqueous gels: agar-agar (10 wt %) (1) and PAA (20 wt %) (2),
as well as the equilibrium swollen PDMS network in heptane (3).
All curves show an exothermic effect at room temperature. For the agar-agar gel, there is also an endothermic effect at 80 °C, which is associated with the gel melting and its transition to a solution.
For the thermally irreversible PDMS and PAA gels, there are no endothermic effects associated with the gel melting, but the exothermic effects are manifested at room temperature. The reasons for the detected exothermic effects are the subject of the present work.
It should be noted that the magnitude of the thermal effect determined by the DSC method under nonequilibrium conditions depends on the heating rate. In order to determine the effect of heating rate on the characteristics of thermal effects, we obtained the DSC curves of the thermally reversible agar-agar gels at different heating rates. Figure 2 shows the change in the characteristics of the exothermic effects depending on the heating rate.
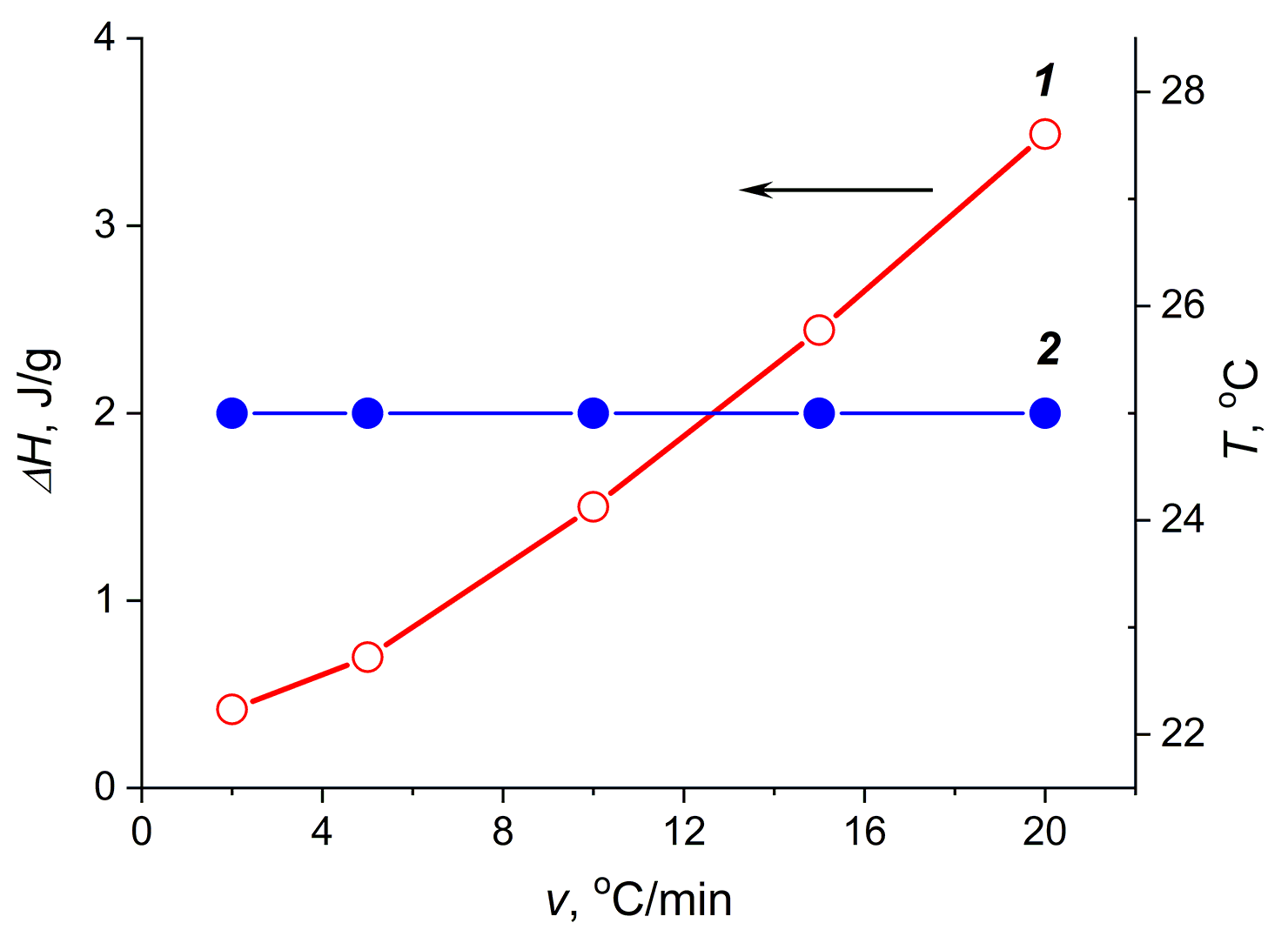
Figure 2. Dependences of ΔH of the exothermic effects of the agar-agar aqueous gels (10 wt %) (1)
and the maximum of the exothermic peak on the DSC curve (2) on the heating rate.
As can be seen from Fig. 2, the magnitude of the thermal effect increases with the increasing heating rate, and the position of the maximum of the exothermic effect on the temperature scale does not change. In our subsequent experiments, we heated and cooled the gels under investigation at a rate of 10 °C/min.
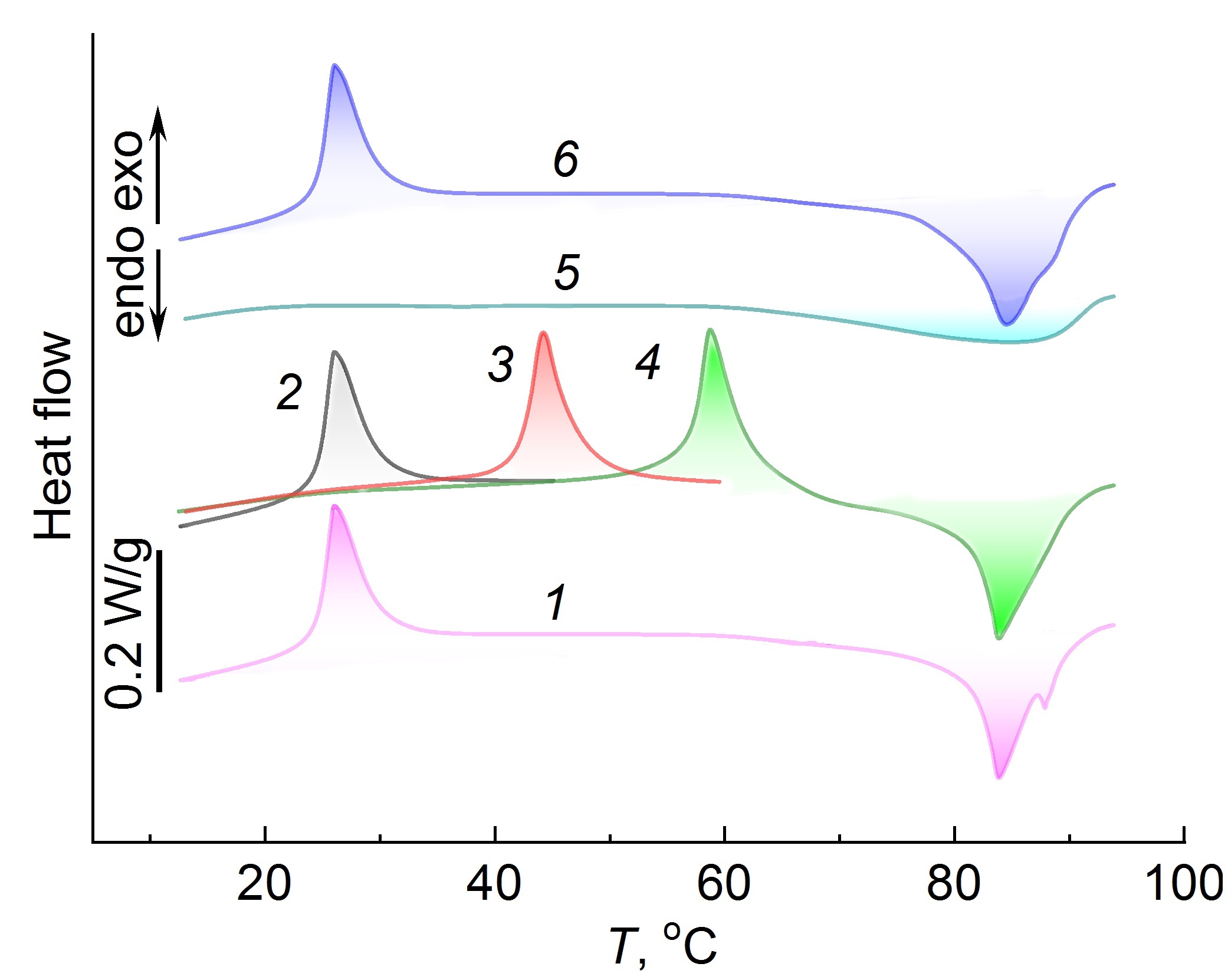
The specificity of the manifestation of the exothermic effect in thermally reversible gels was studied using the example of the agar-agar aqueous gels. Figure 3 presents the DSC curves of the agar-agar aqueous gel with different temperature prehistories. When heated from 5 to 95 °C (Fig. 3, curve 1), two temperature effects were observed: an exothermic effect at room temperature and an endothermic effect associated with the gel melting. If we change the experimental conditions and heat not until the gel melts, but to a certain intermediate temperature, located in the interval between exothermic and endothermic effects (curve 2), then after heating and cooling of the gel to 5 °C and secondary heating, the exothermic effect, which appeared earlier at room temperature, shifts to higher temperatures and appears at a temperature that is close to the final temperature of the first heating (Fig. 3, curve 3). After the third heating–cooling cycle (curve 4), the exothermic effect again shifts to a temperature region close to the temperature at the end of the second heating–cooling cycle. Further heating of the gel to 95 °C leads to its melting, which is accompanied by an endothermic effect. After melting and cooling to 5 °C, upon repeated heating (Fig. 3, curve 4), the exothermic effect does not appear on the DSC curve; there is only a diffuse endothermic effect at ca. 95 °C, which corresponds to the gel melting. Only after cooling of the melt and its storage at 5 °C for 2 weeks, when heated, the exothermic effect is recovered at room temperature (Fig. 3, curve 6), and during subsequent heating–cooling cycles its location on the temperature scale depends on the previous temperature history, as was shown above.
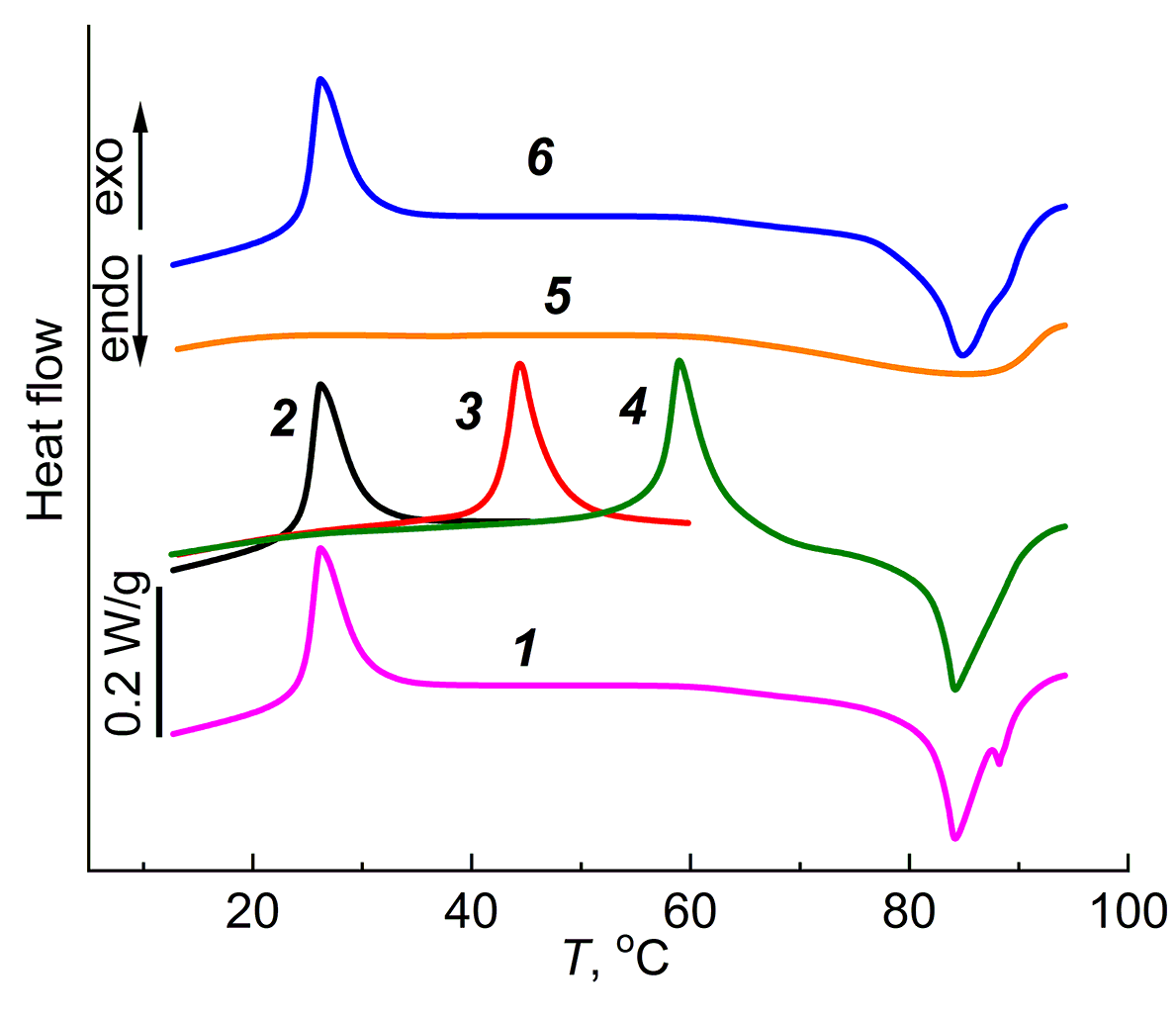
Figure 3. DSC curves of the agar-agar aqueous gel (10 wt %) with different thermal prehistory: heating from 5 to 100 °C (1),
heating from 5 to 45 °C (2), second heating from 5 to 60 °C (3), third heating from 5 to 100 °C (4), heating (after the gel melting) from 5 to 95 °C (5),
heating from 5 to 95 °C (after the gel melting and its storage at 5 °C for 2 weeks).
Note that the value of ΔH of the exothermic effect during the first, second and third heating cycles practically does not change, which indicates the identity of the processes occurring in the gel and causing the exothermic effect during each subsequent heating–cooling cycle.
Thus, the discovered exothermic effect is like a jumping effect, and we can predict the temperature of its manifestation in advance. Similar dependences were obtained for the aqueous agar-agar gels at different concentrations. Figure 4 shows the concentration dependences of ΔH of the exothermic effect for the agar-agar gels upon heating from 5 to 45 °C.
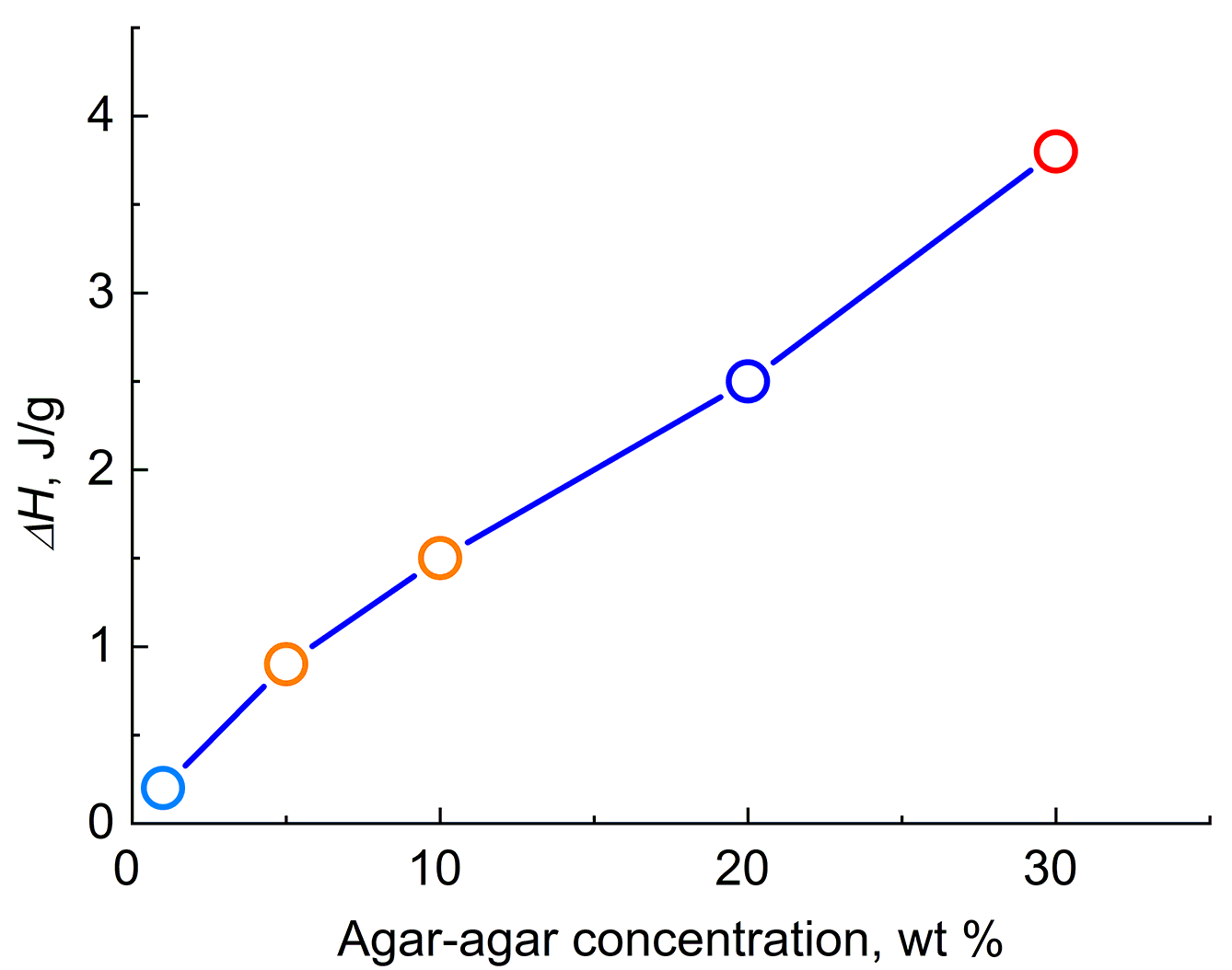
Figure 4. Concentration dependence of ΔH of the exothermic effect for the agar-agar gels.
As can be seen from Fig. 4, an increase in the concentration of agar-agar in the gel leads to an increase in the value of ΔH.
To better understand the reasons for the jumping exothermic effect, the DSC curves of the thermally irreversible aqueous gels of cross-linked PAA were considered. In the PAA gels, the cross-linking extent was regulated by the amount of MBA introduced into the reaction mixture. In this type of gels, there should be no thermal effects at positive temperatures, since the spatial network is formed by chemical bonds and can only be destroyed when the polymer matrix decomposes.
The cross-linking extent was characterized by the molar mass of a chain between the network cross-links (Mc), which was determined from the results of measuring the equilibrium swelling of the PAA gels in water. The value of Mc was calculated by the modified Flory–Rehner equation [12, 13], which is usually used to calculate Mc in polymer networks obtained in solutions.
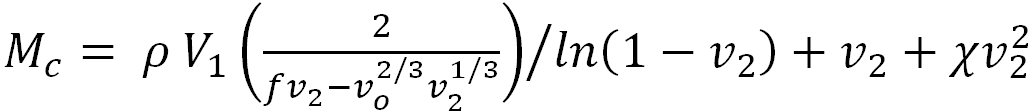 |
, | (1) |
where V1 is the molar volume of the solvent, ρ is the network density, υ0 is the volume fraction of the polymer in the solution at the moment of cross-linking, υ2 is the volume fraction of the polymer in the equilibrium swollen gel, f is the functionality of the network cross-links, i.e., the number of chains emerging from one cross-link, χ is the polymer–solvent interaction parameter, which is equal to 0.492 for the PAA–water system [14]. The volume fraction of the polymer in the equilibrium swollen gel was determined using the value of the polymer mass fraction in the equilibrium swollen gel w2, according to the following equation
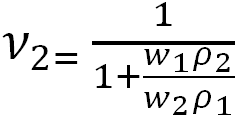 |
, | (2) |
where w1 and w2 are the mass fractions of the solvent and polymer in the equilibrium swollen gel, ρ1 and ρ2 are the densities of the solvent and polymer. The results of the calculations are presented in Table 1.
Table 1. Thermal characteristics of the PAA gels with different MBA contents in consecutive heating–cooling cycles
|
MBA content,
wt %
|
Mc
|
First cycle,
heating range 5–45 °С
|
Second cycle,
heating range 5–60 °С
|
Third cycle,
heating range 5–75 °С
|
Fourth cycle,
heating range 5–90 °С
|
||||
|
Tm, °С
|
∆Н, J/g
|
Tm, °С
|
∆Н, J/g
|
Tm, °С
|
∆Н, J/g
|
Tm, °С
|
∆Н, J/g
|
||
|
2
|
10500
|
19
|
0.2
|
34
|
0.35
|
45
|
0.37
|
55
|
0.28
|
|
4
|
3700
|
23
|
1.2
|
39
|
1.4
|
50
|
1.5
|
68
|
1.4
|
|
8
|
1400
|
25
|
5.5
|
41
|
5.1
|
55
|
5.3
|
70
|
5.4
|
|
20
|
750
|
17
|
1.1
|
28
|
1.2
|
38
|
1.7
|
46
|
1.9
|
Thermally irreversible PAA chemical gels
Like the agar-agar gels, the PAA gels were subjected to several heating–cooling cycles. Figure 5 shows the DSC curves of the 20 wt % PAA gels cross-linked with different amounts of MBA for four heating–cooling cycles. The first cycle started at 5 °C and stopped at 45 °C, followed by cooling the polymer to 5 °C. The second cycle corresponds to heating from 5 °C to 60 °C. As can be seen from Fig. 5, during the first heating, the exothermic effect appears at room temperature; during the second heating, the exothermic effect appears in the temperature region at the end of the first heating.
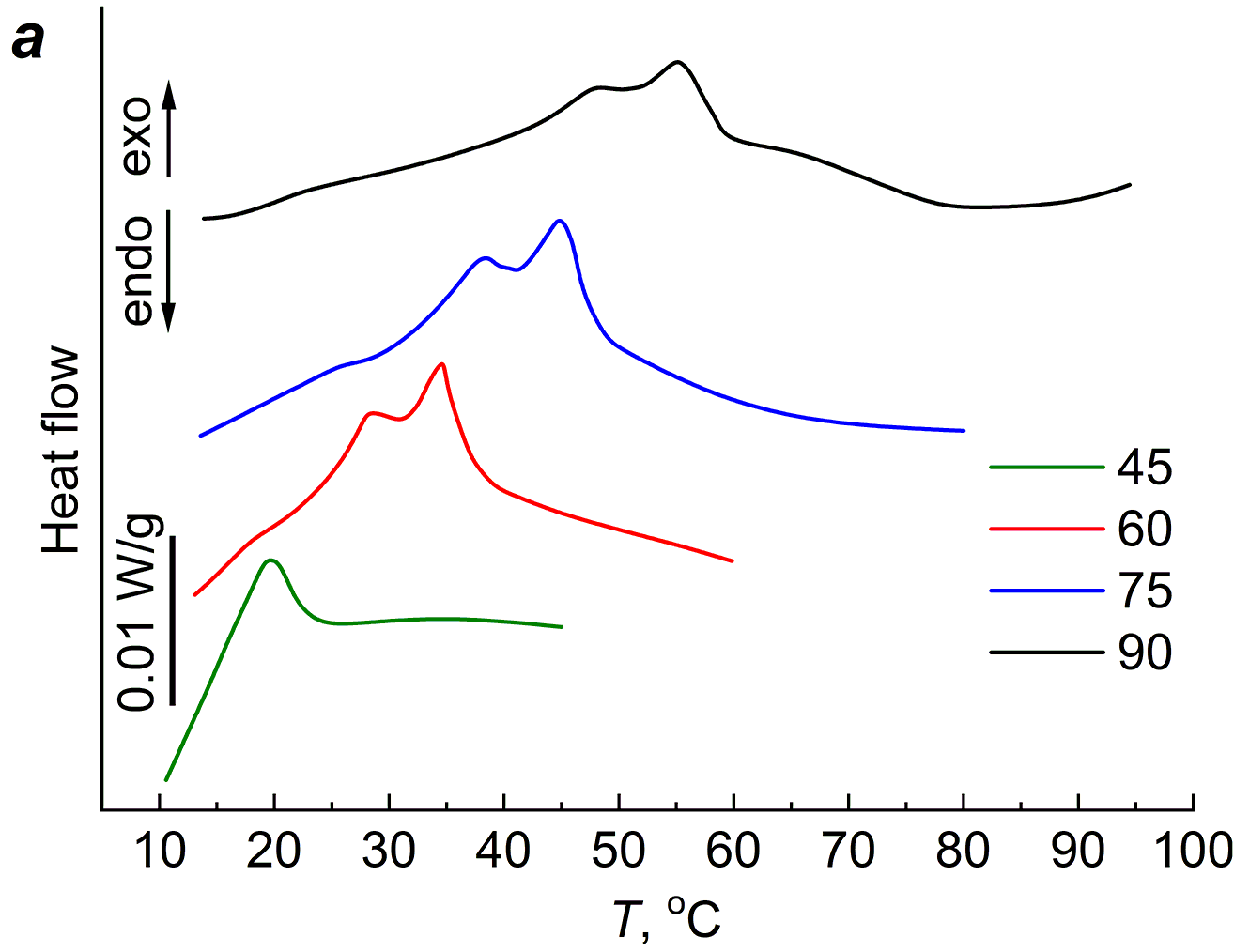
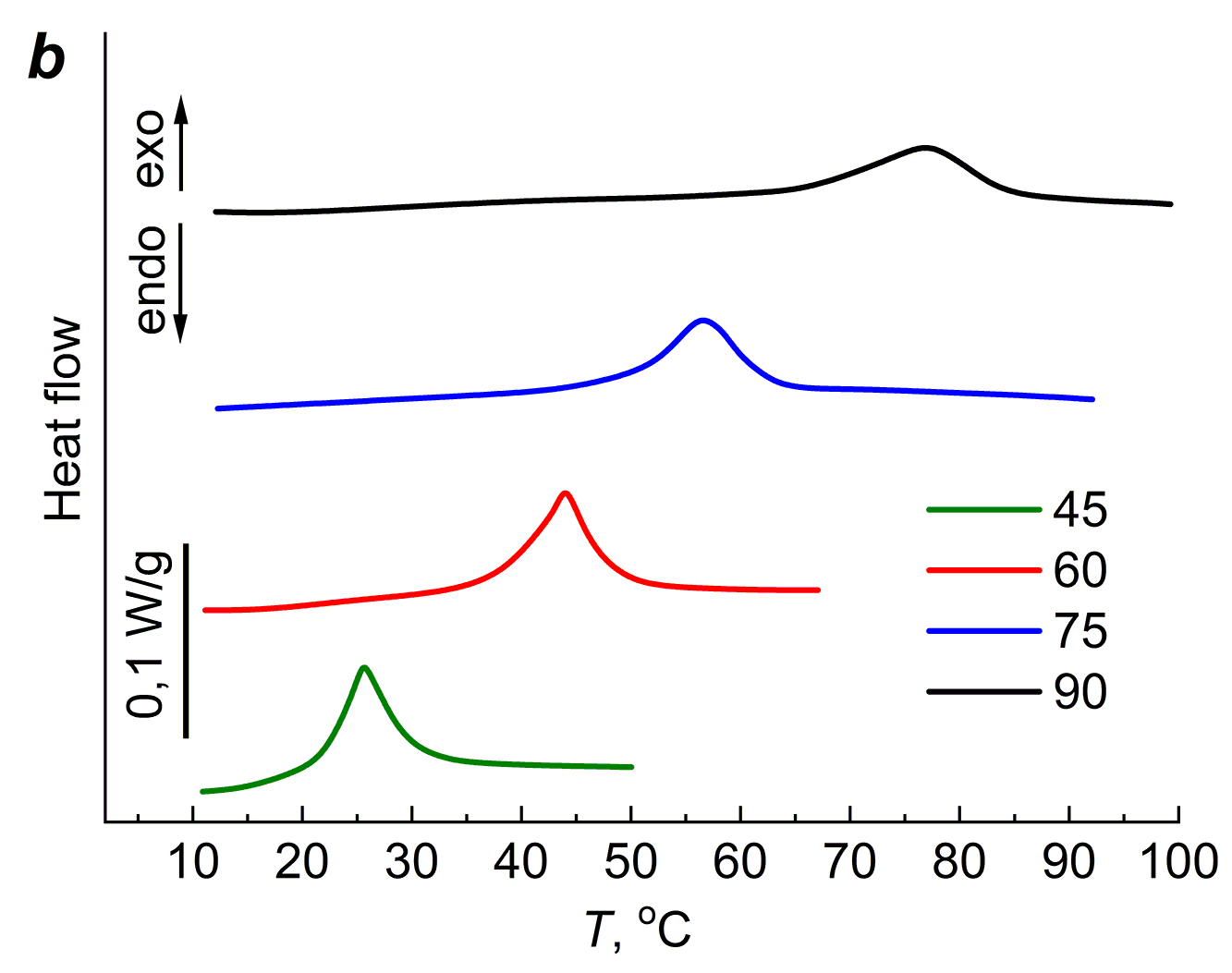

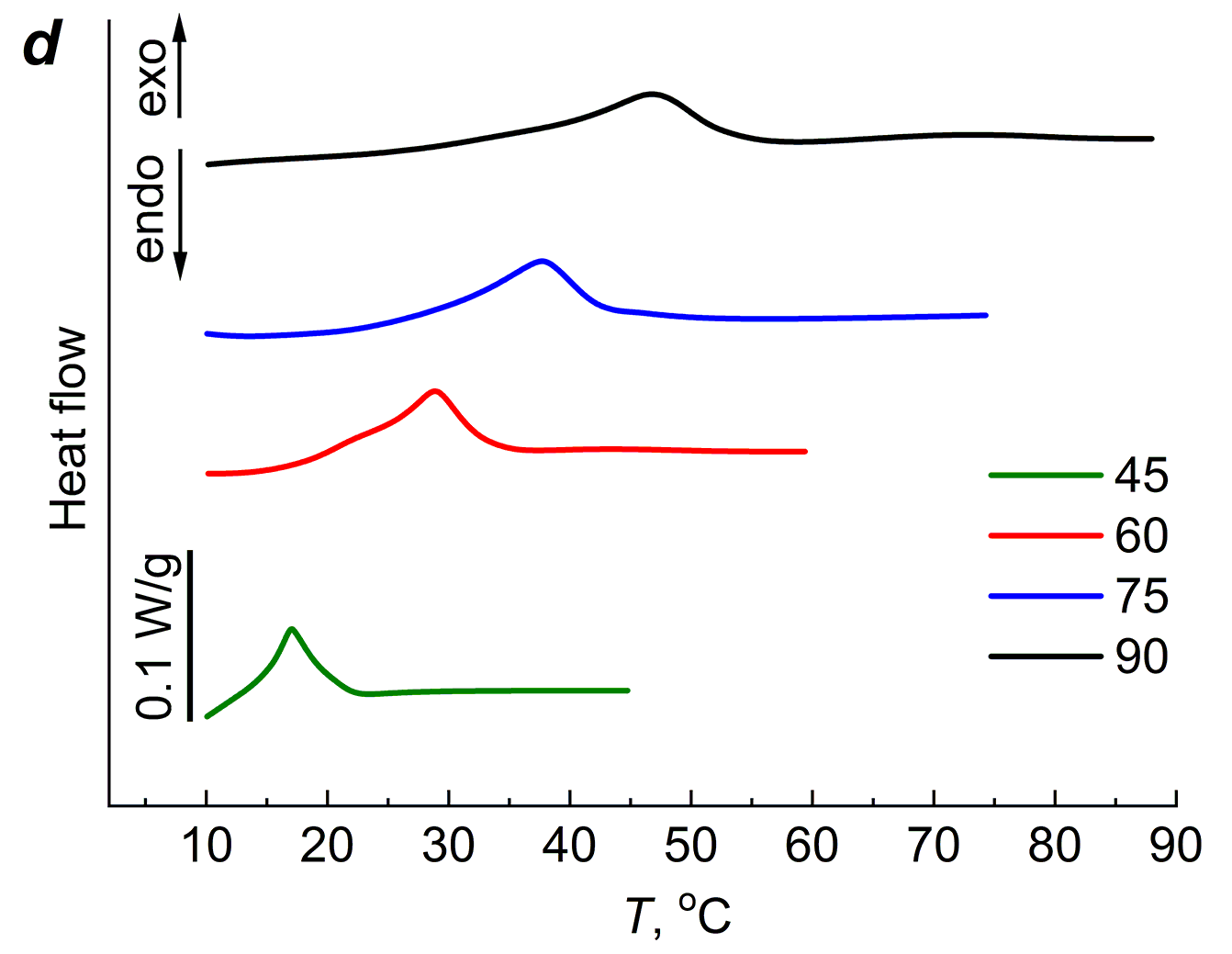
Figure 5. DSC curves of the aqueous PAA gels cross-linked with 2 (a), 4 (b), 8 (c) and 20 (d) wt % of MBA.
The third heating cycle started at 5 °C and continued until 75 °C; the exothermic effect, as in the first two cycles, moved to the temperature region at the end of the second cycle. After cooling to 5 °C, the fourth cycle started, during which heating was carried out to 90 °C. As can be seen from Fig. 5, regardless of the amount of MBA, which determines the cross-linking extent of the gel network, the exothermic effect is present in all four cycles.
The cross-linking extent affects the magnitude and position of the maximum of ΔH. As can be seen from Table 1, the value of ΔH changes extremely with increasing cross-linking of the gel network; the maximum values ΔHm and its position on the temperature scale Tm are observed for the gel containing 8 wt % of MBA, which corresponds to Mc = 1400.
Thus, the cross-linking extent affects the magnitude and temperature range of manifestation of the exothermic effect in the PAA gels. It should be noted that even in highly concentrated aqueous solutions of linear PAA, there is no exothermic effect.
Non-aqueous PDMS gels
PDMS gels are chemically cross-linked (so-called model) networks, in which the molar mass of a cross-linking unit is determined by the molar mass of the cross-linked oligomer. The gels were prepared by swelling the cross-linked polymer, obtained by the block synthesis, in various solvents to an equilibrium state. The equilibrium swollen PDMS gels in heptane and m-xylene were studied.
In the equilibrium swollen gels with a fine network, obtained by swelling of the cross-linked PDMS (Mn = 550), no exothermic effect was detected, while in the gels with a rarer spatial network (Mn = 18000), the exothermic effect appears at room temperature, similar to the aqueous gels of physical and chemical nature. The equilibrium swollen PDMS gels in heptane and m-xylene were chosen as the research objects for studying the jumping exothermic effect in PDMS gels. Figures 6 and 7 show the DSC curves of these gels.
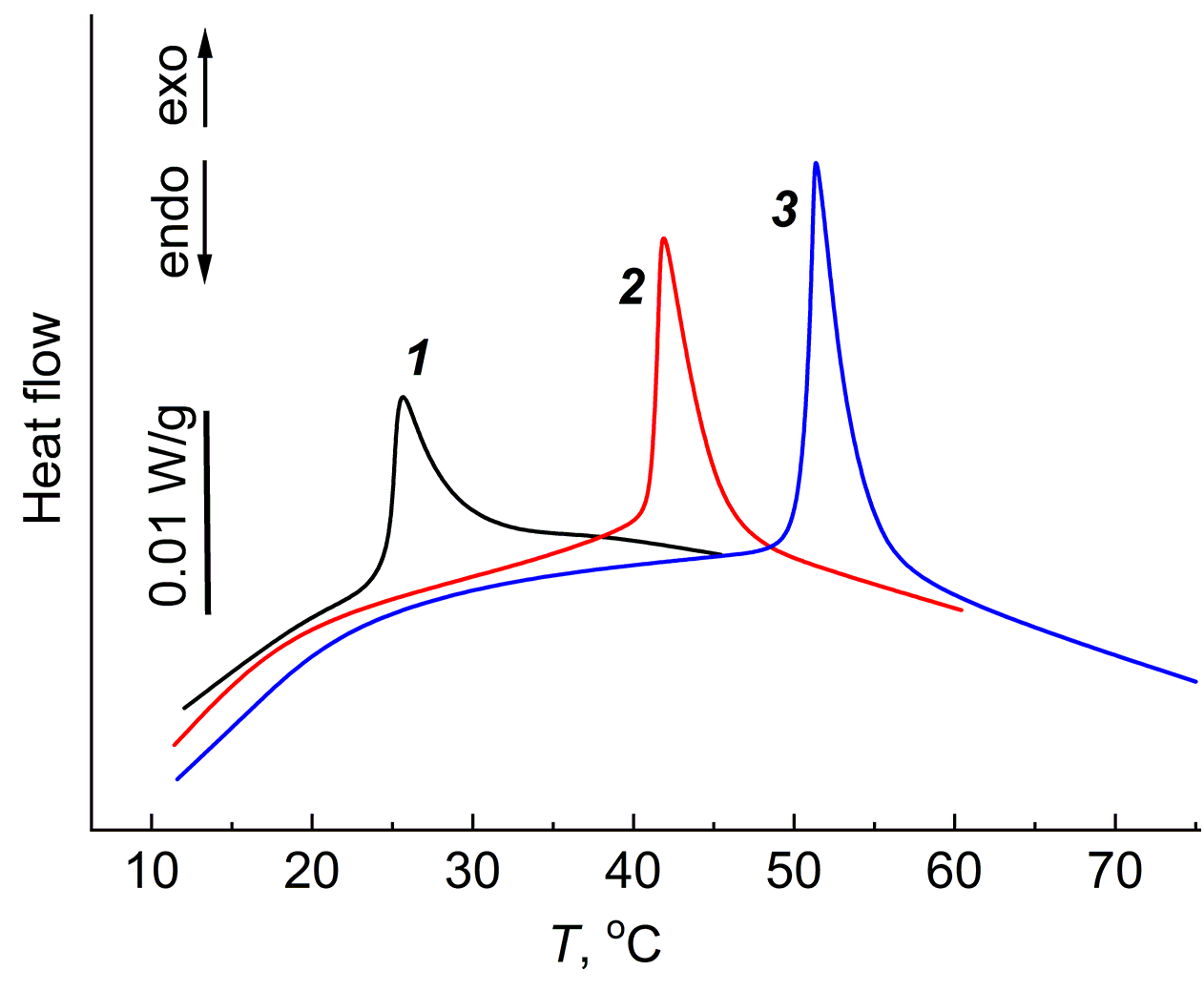
Figure 6. DSC curves of the PDMS gels equilibrium swollen in heptane. Heating ranges: 5–45 °C (1), 5–60 °C (2), 5–75 °C (3).
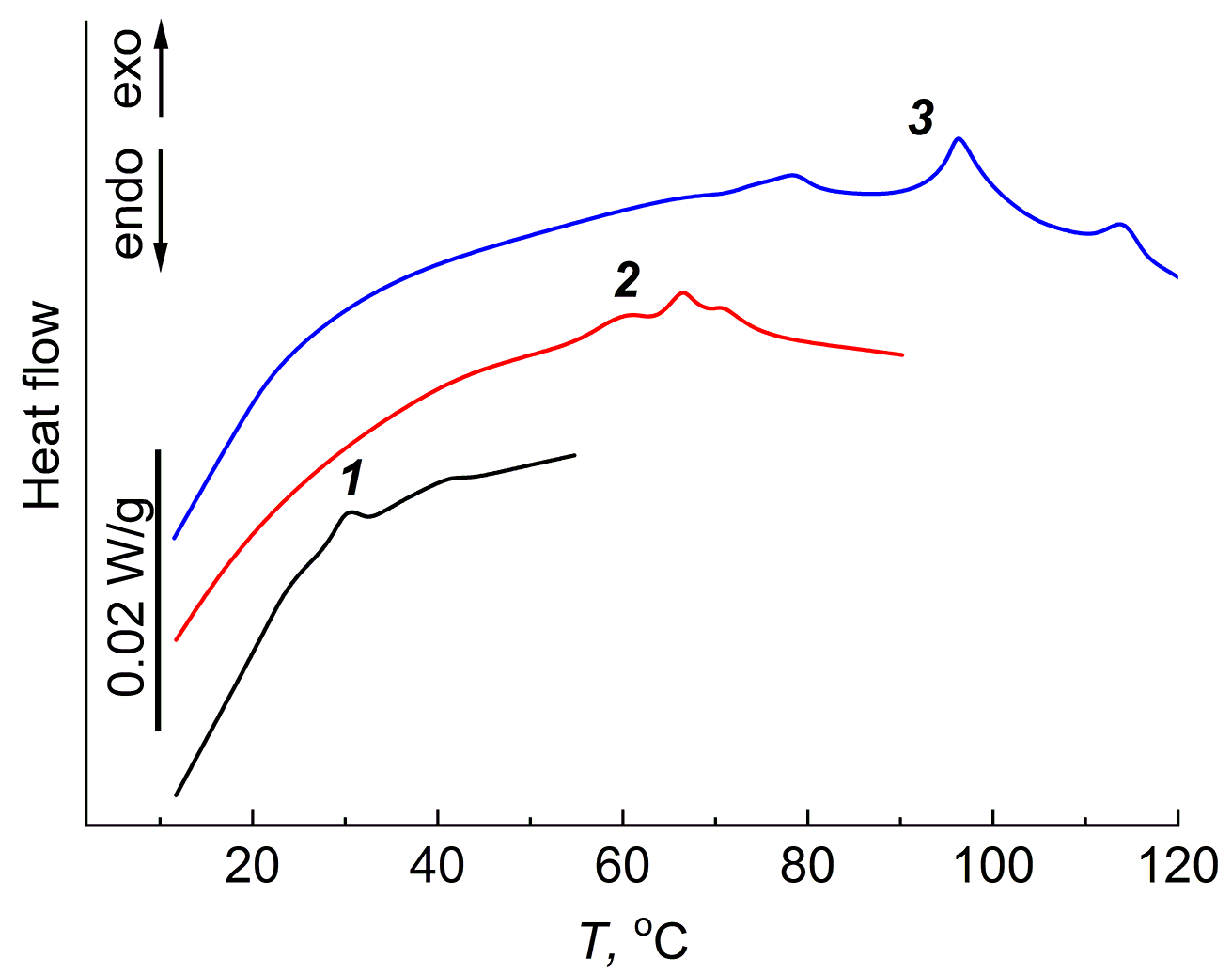
Figure 7. DSC curves of the PDMS gels equilibrium swollen in m-xylene. Heating ranges: 5–55 °C (1), 5–90 °C (2), 5–120 °C (3).
In heptane, the exothermic effects appeared in each heating–cooling cycle, while the value of ΔH was almost independent of the heating cycle order.
For the PDMS gels swollen in m-xylene, the exothermic effect was observed in a wide temperature range from 5 to 120 °C. The exothermic effect remained after the second and third heating, and the value of ΔH gradually increased, reaching the highest magnitude during the third heating. A possible reason for the observed increase in ΔH may be improvement of the thermodynamic quality of the solvent with increasing temperature of the experiment completion.
Table 2 shows the values of ΔH and the temperatures corresponding to the maximum of ΔH for three heating–cooling cycles in both solvents.
Table 2. Parameters of the exothermic effect for the PDMS networks swollen in heptane and m-xylene
|
Heating cycle
|
Heating range
|
Heptane
|
m-Xylene
|
||
|
∆Н, J/g
|
Тpeak, °С
|
∆Н, J/g
|
Тpeak, °Сa
|
||
|
1
|
5–45
|
0.27
|
25
|
|
|
|
2
|
5–60
|
0.23
|
41
|
|
|
|
3
|
5–75
|
0.28
|
51
|
|
|
|
1
|
5–55
|
|
|
0.35
|
30
|
|
2
|
5–90
|
|
|
0.41
|
66
|
|
3
|
5–120
|
|
|
0.77
|
96
|
| a the maximum temperature of the most intensive peak. |
The appearance of the exothermic effect in siloxane gels containing non-aqueous solvents of various thermodynamic qualities indicates that the detected exothermic effect is of a general nature for polymer gels, and the cross-linking extent of the gel network plays an important role in the manifestation of this exothermic effect.
Discussion
As can be seen from the results presented, for any gels, regardless of their nature, the jumping exothermic effect appears on the DSC curves, the position of which on the temperature scale is not constant and depends on the temperature prehistory of the gel. It is likely that the reasons for the appearance of this exothermic effect are the same for all types of gels, so the discussion of the results below is of a general character.
The exothermic effect in polymer–solvent systems is usually observed at the first stage of swelling of the polymer in a low-molecular liquid, when the solvent diffusion into the polymer leads to the solvation of macromolecules [2]. This stage is characterized by the release of heat and ordering of the arrangement of the solvent molecules around the polymer macromolecules, as a result of which the system entropy at the first stage of dissolution usually even reduces. Most likely, it is this process of the solvation of free unbound solvent molecules that causes the observed exothermic effect. An increase in the value of ΔH in the agar-agar gels with the increasing polymer concentration is likely to be associated with an increase in the number of solvated agarose macromolecules. The limited amount of free solvent and the spatial network structure hamper complete dissolution of the polymer, so the solvation is limited. The solvation of macromolecules depends on the structure of the solute and solvent. It is large when all the macromolecule groups are solvated, and small when only some groups are solvated. On this basis, good and bad solvents for polymers are distinguished.
In more concentrated solutions, the solvation of polymer macromolecules may be incomplete, and upon dilution, further solvation will occur, accompanied by an increase in the amount of heat released. In equilibrium swollen gels, the solvation is complete under given thermodynamic conditions. Changes in the thermodynamic conditions lead to changes in the solvation processes [2].
Based on the assumption that the exothermic effect in gels is due to the solvation of polymer macromolecules by the solvent, the above DSC results can be explained. The solvation and ordering of solvent molecules are possible only if there is free (non-solvated) solvent in the gel that can additionally solvate macromolecules.
The appearance of the exothermic effect at room temperature during the first heating cycle may be associated with an increase in the mobility of both the macromolecules themselves and the solvent molecules with an initial temperature rise from 5 °C. An increase in the temperature weakens the specific interactions between macromolecules and increases their mobility and solvating ability. The changed thermodynamic conditions promote the additional solvation and orientation of the free solvent, which leads to the release of heat. During the solvation process, the entropy of the system decreases due to the orientation of the solvent molecules near the macromolecules. A decrease in the entropy complicates conformational changes of the polymer macromolecules and contributes to the freezing of the existing system of specific interactions in the gel. Thus, during the first heating, a new quasi-equilibrium system of bound solvated macromolecules is formed that corresponds to a higher temperature. After completion of the first heating (up to 45 °C), the gel is again exposed to temperature and cooled a second time to 5 °C. During the cooling process, the system of the solvated macromolecules, formed during the first heating, is preserved, since no thermal effects appear during cooling. The system of the solvated macromolecules, formed during the first heating, again undergoes conformational changes during the second heating, which starts at the temperature slightly higher than the temperature at the end of the first heating. Subsequent heating is accompanied by the additional solvation of macromolecules, as indicated by the observed exothermic effect. During the third heating, the polymer macromolecules already contain an additional amount of solvent, solvated during the first and second heating stages. Thus, a peculiar enrichment of the macromolecules with the bound solvent, which is quite firmly connected with the macromolecules included in the gel network, takes place.
The influence of the cross-linking extent on the jumping exothermic effect can be followed using the examples of the PDMS and PAA gels. In the swollen siloxane networks, the exothermic effect is observed in gels with the molar mass of cross-linking unit of 18000, and in the gels with a denser network, the exothermic effect is absent. It should be noted that there is a certain region of cross-linking extent in which the solvation is most fully manifested, as evidenced by the largest value of the exothermic effect ΔH in the PAA gels cross-linked with 8 wt % of MBA.
Based on the results of this study, it can be concluded that the main reason for the occurrence of the jumping exothermic effect is associated with the network structure of a gel, which limits the complete dissolution of the polymer and its transition to the dissolved state. The spatial network that exists in the polymer gel is capable of holding a large amount of solvent, tens and hundreds of times greater than the amount of the polymer. Naturally, in such a system, the solvent has varying degrees of bonding with the polymer. It is precisely this solvent, free or weakly bound to the polymer, that serves as the driving force behind the occurrence of the jumping exothermic effect. In a non-crosslinked polymer, all the solvent included in the polymer is bound, since the macromolecule takes on a certain conformation in solution, depending on the polymer–solvent interaction parameter χ. In a gel, the network imposes certain restrictions on the mobility of cross-linking units. Without external influence, the structure in a polymer gel, especially in a chemical one, is equilibrium. The external temperature effect disrupts the system of intra- and intermolecular specific interactions, existing in the gel, and leads to an increase in the mobility of macromolecules, which contributes to the formation of a new system of bonds between the solvated macromolecules and a free solvent. The appearance of the jumping exothermic effect indicates the emergence of some additional ordered structure, which is formed in a new temperature range. This quasi-equilibrium structure is quite resistant to the temperature impact and is transformed into a new system at the temperatures exceeding the previous heating–cooling cycle; it is obvious that the gel network has a certain stabilizing effect on the newly formed bond system. Figure 8 shows the DSC curves of the PAA gel heated to 60 °C, then cooled and kept at room temperature for 14 days, then heated again. As can be seen from this figure, after exposure, the jumping exothermic effect is restored at the previous temperature, which indicates a certain degree of stability of the new structural arrangement of the solvated macromolecules and solvent included in the network. In physical gels, after the gel melts and goes into solution, the memory of previous structural metamorphoses is lost.
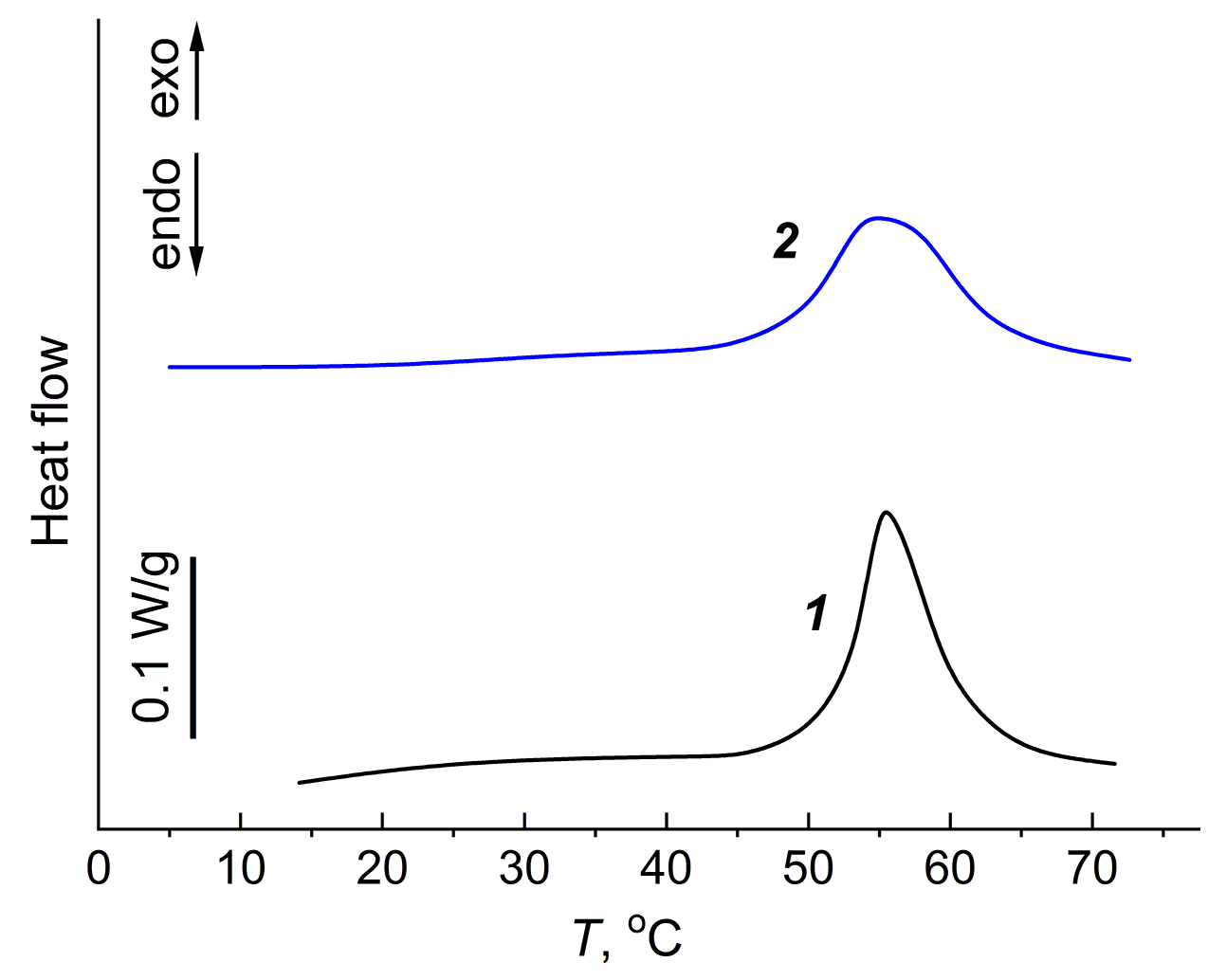
Figure 8. DSC curves of the PAA gel cross-linked with 8 wt % of MBA heated to 60 °C (1) and the same sample heated repeatedly
after exposure for 14 days at room temperature (2).
Conclusions
As a result of this study, the exothermic effect that appears only in polymer gels was characterized. The discovered jumping exothermic effect occurs upon heating of the gels of various natures. Its manifestation unambiguously indicates the presence of a spatial network structure and can serve as an additional criterion that confirms the gel state. The temperature at which the jumping exothermic effect is manifested does not depend on the nature, heating rate, or concentration of the polymer in the gel. The magnitude and temperature range of manifestation of the jumping exothermic effect are not constant and are determined by the temperature prehistory of its formation. The network structure of the gel must contain a sufficient amount of free solvent and not limit the mobility of cross-linking units of macromolecules. Since the discovered phenomenon is common for a large number of gels, it can be attributed to the changes in exchange processes between the free solvent and the gel network, which are of a relaxation nature.
Acknowledgements
This work was performed with financial support from the Ministry of Science and Higher Education of the Russian Federation (agreement no. 075-00277-24-00) using the equipment of the Center for Molecular Composition Studies of INEOS RAS.
References
- K. te Nijenhuis, Thermoreversible Networks. Viscoelastic Properties and Structure of Gels, Berlin, Heidelberg, Springer, Adv. Polym. Sci., 1997, vol. 130. DOI: 10.1007/BFb0008699
- A. A. Tager, Physical Chemistry of Polymers, Khimiya, Moscow, 1978 (in Russian).
- S. P. Papkov, Gel-Like State of Polymers, Khimiya, Moscow, 1974 (in Russian).
- T. Tanaka, in: Encyclopedia of Polymer Science and Engineering, A. Klingsberg, P. Piccinini (Eds.), Wiley, New York, 1985, ch. 7, pp. 514–520.
- L. H. Sperling, Introduction to Physical Polymer Science, 2nd ed., New York, Wiley, 1992.
- L. Z. Rogovina, V. G. Vasil'ev, E. E. Braudo, Polym. Sci., Ser. C, 2008, 50, 85–92. DOI: 10.1134/S1811238208010050
- M. Watase, K. Nishinari, A. H. Clarks, S. B. Ross-Murphy, Macromolecules, 1989, 22, 1196–1201. DOI: 10.1021/ma00193a034
- M. Watase, K. Nishinari, Makromol. Chem., 1987, 188, 1177–1l86. DOI: 10.1002/macp.1987.021880520
- V. G. Vasil'ev, M. I. Buzin, G. G. Nikiforova, N. M. Belomoina, E. G. Bulycheva, V. S. Papkov, Dokl. Phys. Chem., 2014, 458, 149–152. DOI: 10.1134/S0012501614100029
- L. A. Osterman, Methods for Studying Proteins and Nucleic Acids: Electrophoresis and Ultracentrifugation (a Practical Guide), Nauka, Moscow, 1981 (in Russian).
- V. V. Severny, R. M. Minasyan, I. A. Makarenko, N. M. Bizyukova, Vysokomol. Soedin., Ser. A, 1976, 18, 1276–1281.
- P. J. Flory, Principles of Polymer Chemistry, Cornell Univ. Press, Ithaca, New York, 1953.
- P. J. Flory, J. Rehner, J. Chem. Phys., 1943, 11, 512–520. DOI: 10.1063/1.1723791
- Polymer Handbook, 4th ed., J. Brandrup, E. H. Immergut, E. A. Grulke (Eds.), Wiley, New York, 1999.





