2023 Volume 6 Issue 4 (Published 20 July 2024)
|
INEOS OPEN, 2023, 6 (4), 97–105 Journal of Nesmeyanov Institute of Organoelement Compounds |
|
Sulfonated Polynaphthoyleneimides and Polybenzimidazoles for Hydrogen–Air Fuel Cells
Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, str. 1, Moscow, 119334 Russia
Corresponding author: K. M. Skupov e-mail: kskupov@ineos.ac.ru
Received 9 April 2024; accepted 14 May 2024
Abstract
This review focuses on the synthesis and investigation of fluorine-free heterocyclic polyheteroarylenes (PHAs) which can be used as proton exchange membranes (PEMs) in hydrogen–air fuel cells at 60–200 °C. Below 100 °C, sulfonated PHAs are typically employed as PEMs. Among them, polynaphthoyleneimides with SO3H substituents have received significant attention. At higher temperatures (>120 °C), polybenzimidazole-based PEMs doped with phosphoric acid exhibit superior performance.
Key words: LT–PEMFC, HT–PEMFC, sulfonated polynaphthoyleneimide, polybenzimidazole.
Introduction
Today, one of the most important research problems around the world is to find an alternative to fossil fuels. The challenge is related to the growing demand for energy consumption, as well as reducing of harmful emissions into the atmosphere, which, in turn, affect global warming and climate change. Fuel cell power plants represent one of the most versatile examples of clean energy generation suitable for both stationary and mobile applications [1].
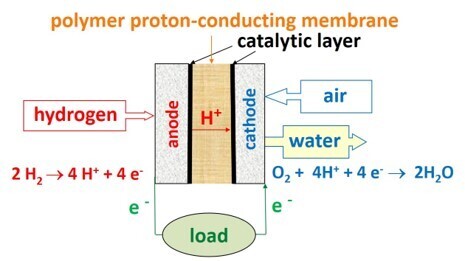
Fuel cells (FCs) convert chemical energy into electricity and heat using an electrochemical process. The fuel can be hydrogen, methanol, syngas, methane, etc. Typically, fuel cells are classified according to the type of an electrolyte used in the membrane electrode assembly (MEA). Polymer-electrolyte (or proton-exchange) membrane fuel cells (PEMFCs) are preferred among all types due to their simple structure and high efficiency [2–4]. In these fuel cells, the redox processes take place by supplying hydrogen (or methanol) to the anode and air (or oxygen) to the cathode. In this case, a MEA is an assembly consisting of two porous electrically conductive carbon gas diffusion electrodes Pt/C (anode and cathode) and a thin gas barrier polymer proton-conducting membrane. Kirubakaran et al. reviewed [5] the comparative characteristics (efficiency, productivity, etc.) for the operation of PEMFC and other power sources.
Fluorine-free heterocyclic PHAs can be used as PEMs in air–hydrogen fuel cells at 60–200 °C. For operating temperatures below 100 °C, the PEMs based on sulfonated PHAs are used [2, 3]. At higher temperatures (>120 °C), the PEMs based on polybenzimidazoles (PBIs) and doped with phosphoric acid are among the best ones [4].
Over the past two decades, a significant number of studies and reviews have been focused on the above-mentioned polymers, but not all interesting and significant works have been adequately covered. The present review was not intended to cover all fluorine-free PHA-based membranes for PEMFC. Instead, it aims to notice important achievements and highlight the most significant approaches in the field of PEMFC membrane production, with the ultimate goal of overcoming current PEMFC MEA challenges. Therefore, the authors tried to focus on some deficient information from previous years and entirely new data which were not included in other reviews.
The most commonly used polymer-electrolyte membrane for low-temperature polymer-electrolyte membrane fuel cells (LT–PEMFCs) is the sulfonated perfluorinated Nafion® membrane (DuPont, Wilmington, DE, USA). This material exhibits excellent mechanical properties and proton conductivity (approximately 100 mS/cm at room temperature when hydrated). Proton transfer in the Nafion® membrane occurs by the Grotthuss mechanism, which involves the transfer of protons through the formation and cleavage of hydrogen bonds in an aqueous medium. Therefore, the Nafion® perfluorinated PEM ensures operation of FC only at sufficient humidity (≥80%), which reduces the overall FC efficiency and complicates its design. Another drawback of the Nafion®-based LT–PEMFC is the requirement for hydrogen purification (99.999%), mainly from carbon monoxide, which is produced by steam reforming of hydrocarbons. CO can be adsorbed onto the surface of the platinum catalyst particles on the electrodes, reducing the performance of the FCs. Additionally, the service life of Nafion® is short (under voltage cycling conditions), its cost is high and disposal is difficult due to fluorine content [6–10].
Therefore, significant research efforts have been directed to find new fluorine-free aromatic polymers as a possible alternative to the Nafion® membrane [11, 12].
The proton-conducting membranes based on polybenzimidazole, which are a polymer-electrolyte complex of the polymer and phosphoric acid, can operate at 140–200 °C (which distinguishes them from the Nafion membranes) and provide high performance of the platinum catalyst as well as its resistance to impurities.
To design a high-performance and efficient PBI-based membrane doped with phosphoric acid for HT–PEMFC, it is essential to maintain a balanced ratio between the amount of phosphoric acid in the membrane and its mechanical properties. Higher phosphoric acid content increases proton conductivity but also reduces the mechanical stability. The phosphoric acid acts as a plasticizer, helping to improve the mechanical properties of the membrane. One of the key techniques used to enhance the mechanical strength of a membrane involves the process of ionic or covalent cross-linking of the material, typically using cross-linking agents. Unfortunately, the data regarding membrane proton conductivity and MEA performance are reported under different experimental conditions, which may hamper the correct comparison of the membrane properties. At the same time, the data presented in the review provide sufficient evidence to conclude whether PBI membranes could be used in HT–PEMFC MEA. Currently, the most extensively studied PBI membranes for HT–PEMFC are those based on m-PBI and ABPBI (poly(2,5-benzimidazole)) (Fig. 1).
Figure 1. Chemical structures of m-PBI and ABPBI.
The proton conductivity of a neat undoped PBI-based membrane is ~0 mS/cm, while that of the membrane doped with PBI·5H3PO4 reaches 60 mS/cm at 180 °C [13]. Li et al. [14] demonstrated that the performance of HT–PEMFC MEA at 160 °C using m-PBI Celtec® (BASF, Ludwigshafen am Rhein, Germany) membranes are 0.60 V at 400 mA/cm2 and 0.65 V at 200 mA/cm2. Asensio and Gomez-Romero [15] reported that the proton conductivity of the phosphoric acid-doped membrane at 180 °C in dry conditions reaches 25 mS/cm for ABPBI·2.7H3PO4 and 15 mS/cm for ABPBI·3.0H3PO4, depending on the peculiarities of membrane production. At the same time, at a relative humidity of 30%, the value of proton conductivity for ABPBI·3.0H3PO4 reaches 60 mS/cm at 150 °C. The performance of the ABPBI membrane (FuMA-Tech, Bietigheim-Bissingen, Germany) in the hydrogen–air HT-PEMFC MEA at 160 °C was 0.60–0.63 V at 200 mA/cm2 [16]. As can be seen, the proton conductivity of these membranes does not approach the proton conductivity of phosphoric acid (~500 mS/cm at 155–160 °C) [13].
For LT–PEMFC, new fluorine-free aromatic polymers are of particular interest. For HT–PEMFC, in order to make further progress in the field of PBI membranes, it is necessary to move toward increasing the proton conductivity, while at the same time maintaining or even improving the mechanical strength of the membranes.
Sulfonated polynaphthoyleneimide membranes
From the aforementioned perspective, polyheteroaromatic membranes, specifically those based on polynaphthoyleneimide (PNI) with six-membered imide rings and aromatic sulfonate fragments in a free H+-form, which feature chemical stability and high ionic conductivity at <100 °C, offer considerable benefits. The advantages and disadvantages of sulfonated polynaphthoyleneimide (SPNI)-based membranes have been thoroughly analyzed in the literature [17]. However, it should be noted that the aromatic PNIs based on 1,4,5,8-naphthalenetetracarboxylic acid have not been widely used in recent years due to their insolubility in organic solvents and inaccessibility by a two-step synthetic process through a soluble polyamide acid step, as is the case with polyphthalimide [18]. Significant interest in PNI arose after high-molecular weight soluble film-forming PNIs based on cardo aromatic diamines were obtained at INEOS AS USSR for the first time applying the method of high-temperature catalytic polycondensation in the presence of benzoic acid in a medium of nitrobenzene and phenols [19, 20]. Later, it was shown that the use of a mixture of benzoic acid and benzimidazole as a polycondensation catalyst allows for the production of rigid-rod PNI, which is soluble in phenolic solvents and forms films with unique mechanical properties [21–23]. All film materials based on PNI produced during these years showed thermal, chemical, and radiation resistance, as well as increased strength and durability. Aromatic and heterocyclic rings in PNI form rigid conjugated structures with high glass transition temperatures and strong bonds, which make films based on them resistant to aggressive environments. The aforementioned advantages of polyimides containing six-membered ring structures led to the possibility of PNI functionalization, particularly, with sulfo groups for application as proton-conducting membranes [24–26]. For polymer electrolytes, proton conductivity depends on ion exchange capacity (IEC), water uptake, acidity of ionogenic groups, and membrane morphology. The proton conductivity of non-fluorinated PHAs can be enhanced by sulfonation; however, to achieve higher proton conductivity than that of the Nafion-type membranes, a higher degree of sulfonation is required [27, 28]. Among the well-established PHAs, which are suitable for PEMFC, sulfonated polyimides with six-membered polyimide rings were found to be the most effective ones owing to their exceptional heat resistance, superior mechanical durability, excellent film-forming capabilities, and exceptional chemical stability. These advantages are precisely what is required for polymer-electrolyte membranes which are used in FC [29–37]. It should be noted that, while traditional five-membered sulfonated polyphthalimides are proven to be effective materials and have been studied for many years, their use as proton-conducting membranes in FCs has not been successful [17]. Sulfonated polyphthalimide membranes immersed in water at 60–80 °C became brittle within 10 h due to high sensitivity to hydrolysis, while SPNIs were much more stable and practically did not deteriorate under FC operating conditions (about 3000 h) [32, 37]. It was shown that the primary process of SPNI degradation involves the imide ring opening through hydrolysis under acidic conditions [17].
Polynaphthoyleneimides are typically synthesized via the polycondensation of commercially available 1,4,5,8-naphthalenetetracarboxylic acid dianhydride (NTDA) and various diamines (both sulfonated and non-sulfonated) in a one-step process in phenolic solvents with addition of benzoic acid and triethylamine (or another organic base) as catalysts with a stepwise temperature rise from 80 to 200 °C [18, 38, 39]. Some of the applied diamines are shown in Fig. 2.
Figure 2. Chemical structures of the diamines: 4,4'-diaminobenzidine-2,2'-disulfonic acid (BDSA),
4,4'-diaminodiphenyloxide-2,2'-disulfonic acid (ОDАS), 4,4'-bis-methylenedianthranilic acid (MDAA).
Due to low solubility of the dianhydride monomer NTDA (Fig. 3) and many PNIs based on it in organic solvents, as well as the use of highly toxic phenolic solvents in the synthesis, new binuclear dianhydrides were developed, leading to the creation of SPNIs which are soluble in aprotic solvents [40, 41].
Figure 3. Chemical structures of NTDA (left) and 4,4'-ketodinaphthyl-1,1',8,8'-tetracarboxylic acid dianhydride synthesized at INEOS RAS (right).
Thus, the proton conductivity of a copolyimide derived from 4,4'-ketodinaphthalene-1,1',8,8'-tetracarboxylic dianhydride, which is soluble in DMSO (Fig. 3), was found to be just slightly lower than that of the Nafion membrane. Additionally, the tensile strength of the films decreased by 1.5–3 times after immersion in water and exposure to 130 °C for 300 h. Copolymers, as opposed to homopolymers, based on dinitrile or other carboxylic acid dianhydrides, aromatic diamines containing sulfonic groups and aromatic and aliphatic non-sulfonated amines exhibit greater stability in aqueous solutions due to the reduced number of sulfonic acid groups (and hence a decrease in ion exchange capacity and proton conductivity). It is considered that, for successful operation in PEMFC, the ion exchange capacity of SPNI-based membranes should be ranged within 1.2–2.5 mEq/g [17]. At the same time, by varying the monomers, it is possible not only to affect the solubility but also to incorporate functional groups into the polymer, which can impart new physical and chemical properties. The studies on copolyimides have shown the achievement of high proton conductivity of 200 mS/cm at 100% relative humidity and high temperatures (above 100 °C) from BDSA with non-sulfonated diamine moieties. However, no mention has been made regarding the hydrolytic stability of these materials [42–45]. Lee et al. [46] reported the stability in water of the BDSA-based SPNI for up to 110 h at 80 °C. Other research groups [37, 47] reported on the aging of the developed polynaphthoyleneimide membranes (based on NTDAs, BDSAs, and conventional non-sulfonated diamines). These membranes showed acceptable performance in the PEMFC H2/O2 operating system at 60 °C for over 3000 h. However, their proton conductivity was significantly lower due to their low ion exchange capacity (IEC 1.30 mEq/g). Furthermore, random and block SPNI membranes with an IEC of ≥ 2.0 mEq/g dissolve or undergo fragmentation when exposed to water at elevated temperatures (>50 °C). Perrot et al. [48] showed that sulfonated naphthoyleneimides undergo fragmentation under hydrothermal conditions (130 °C, 170 h) and then recombine to form novel polyimides which are stable under such conditions. Therefore, it is essential to find a balance between the IEC and sufficient stability of the polymeric film in an aqueous medium.
A multitude of different studies were conducted on the hydrolytic stability of SPNI with respect to the membrane morphology and monomer chemical composition [49–61]. The monomer chemical structure affects the ease with which protons or water molecules may interact with the SPNI imide ring. Due to an increase in the electron density on the nitrogen atom of the imide ring caused by the introduction of substituents into the benzene ring, the membrane hydrolytic stability is enhanced. In particular, the presence of a donor substituent at an ortho/para-position of the aromatic ring complicates the process of ring opening and, consequently, enhances the hydrolytic stability of the polymer. A positive impact of the CF3-group at the ortho-position has also been observed; despite its acceptor nature, its hydrophobicity may present challenges for water molecules to approach the carbonyl group [51]. It was noted that the monomer chemical structure influences the membrane morphology, which, in turn, affects water uptake. It is generally assumed that water uptake ensures efficient proton transport according to the Grotthus mechanism (H3O+ diffusion) across the membrane. However, there are three problems associated with water uptake by SPNI. First, excessive swelling of the membrane under operating conditions at high humidity may cause them to peel off from the catalyst layer. This can prevent the occurrence of a three-phase boundary between the reactant gas, proton-exchange membrane, and electrodes (with the catalyst on the surface) and prevent the redox reaction. Second, low levels of water uptake can result in low proton conductivity. Conversely, at high levels, the swollen membrane significantly changes in size, and proton conductivity may also decrease due to a reduction in proton concentration within the polymer matrix. Third, when sulfonic groups are incorporated into polyimides, water absorption capacity increases from 15% to 80%. This may also lead to their partial or total dissolution in water at higher levels of sulfonic groups [52–56]. Water uptake must be optimal for the operation under conditions of high temperatures and low humidity. Therefore, SPNI should be designed to retain water even under low relative humidity conditions and provide high proton conductivity, while, at the same time, avoiding excessive water uptake. A potential solution could be the use of a polymer with high free volume, achieved by incorporating bulky groups, such as cardo groups, into the polymer structure. The introduction of sulfonic groups as side substituents, as well as the presence of bulky substituents in the main chain result in noticeable separation of hydrophilic and hydrophobic microphases [49, 57–61], which, in turn, increases hydrolytic stability. In particular, SPNI with sulfonic acid groups on the side chains showed an improvement in their hydrolytic stability due to higher basicity of the diamine moieties and their microheterogeneity. Using a similar approach, Asano et al. [62] synthesized highly stable sulfonated copolyimides containing aliphatic groups in both their main and side chains in order to effectively enhance hydrolytic stability, without compromising other properties, such as proton conductivity, oxidative stability, and mechanical strength. Under operating conditions, the resulting MEA demonstrated stability for 5000 h (relative humidity 60–90% at 80 °C, with IEC of 1.82 mEq/g). This result indicates sufficient hydrolytic stability and low level of gas permeation through the membrane (Fig. 4) [62].
Figure 4. Structure of a highly stable SPNI with aliphatic groups in both the main and side chains.
A promising approach to achieving high microheterogeneity consists in the synthesis of multiblock copolymers comprising hydrophilic and hydrophobic units. It was reported that a combination of ODAS and MDAA monomers provides improved hydrolytic stability, while maintaining high proton conductivity and excellent mechanical strength [63–66]. The chemical structure of the resulting copolymer consists of hydrophilic flexible ODAS/NTDA units with an IEC value of 3.37 mEq/g, exhibiting high proton conductivity of 100 mS/cm in water even at low temperature. However, these units do not maintain their integrity and may dissolve. In addition, the copolymer contains hydrophobic (presumably less hydrophilic) MDAA/NTDA segments, which could play the role of an internal catalyst in the polymer chain reorganization in the case of its degradation due to hydrolysis. By varying the proportion of hydrophilic and hydrophobic segments, the pathways for water diffusion and, therefore, the transport properties can be altered. It results in a lower dependence of humidity and temperature on proton conductivity, as well as a general decrease in both water uptake and size changes. A membrane with the ODAS/MDAA ratio of 70/30 (or PNIS70/30 with IEC of 2.44 mEq/g) is optimal in terms of chemical strength, proton conductivity, and water self-diffusion coefficient compared with other membranes of this type (Fig. 5).
Figure 5. Sulfonated polynaphthoyleneimide copolymer featuring a combination of monomers ODAS/MDAA (co-PNIS70/30).
co-PNIS70/30 membranes appeared to be promising for the use in both methanol–air [63] and hydrogen–air fuel cells [64–67].
Mobile methanol PEMFCs are anticipated to be utilized in transportation due to their high energy capacity and ease of use as a fuel source. The membranes based on SPNI are promising for specific use in methanol PEMFCs, as they exhibit significantly lower methanol permeability compared to the Nafion and sulfonated polyarylene ether membranes. Thus, a study [63] was conducted on the performance of the MEAs based on co-PNIS70/30 membranes and those based on the Nafion membranes in a methanol–air fuel cell operating at 40 °C. The calculated current density and power density of the methanol–air fuel cell with these membranes at a voltage of 0.3 V were as follows: 1) for Nafion, 73 mA/cm2 and 22 mW/cm2, respectively; 2) for co-PNIS70/30, 54.5 mA/cm2 and 16 mW/cm2, respectively. These findings indicate that the tested co-PNIS70/30 membrane is a promising alternative for use in portable methanol–air fuel cells. It has virtually no drawbacks compared to the Nafion membrane.
In order to increase the membrane stability and ensure proton conductivity, various research groups developed the techniques for cross-linking SPNI or creating meshed structures [63–71].
The specific zirconium cross-linking between the ODAS and MDAA blocks allows the production of self-wetting membranes due to zirconium affinity to water in hydrogen–air fuel cells. A membrane of this type dries more slowly under anhydrous conditions (at elevated temperatures) and maintains its good transport properties for a longer period of time. Additionally, in order to prevent ohmic loss associated with the peeling of the membranes from the catalyst layer [64], a hot press wave is applied at a pressure of 8–13 MPa and 130 °C before initiating the MEA operation. It results in the optimal interphase connection. It was established that the optimal operating temperature for the fuel cells based on co-PNIS70/30 membrane is 60–65 °C. In this case, the maximum power density of the MEA is ~535 mW/cm2 and the operating power density (at 0.6 V) is 415 mW/cm2 (the maximum output power for the MEA with the conventional membrane and catalytic electrode layer pressing is 370 mW/cm2 [66]).
A comparison with the analogous characteristics of the commercially available Nafion membrane reveals that the power density values are almost identical (the difference is ~1%), and the maximal MEA power density for ODASx/MDAA1–x (x = 70) is only ~20% lower (Fig. 6) at 100% humidity of supplied gases (hydrogen and air).
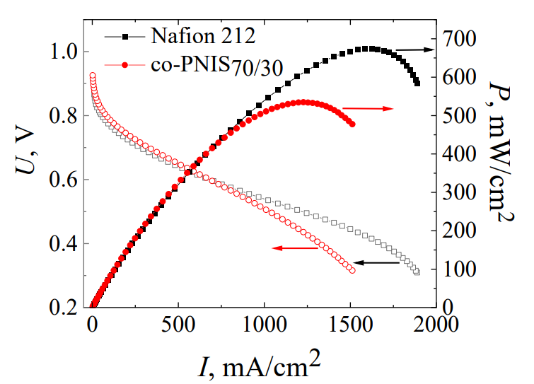
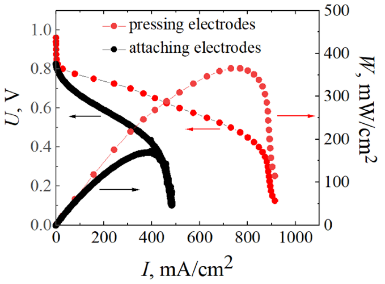
Figure 6. Comparison of the MEA performance for the co-PNIS70/30 membrane (with hot pressing of the membrane and electrode catalytic layer) and the
Nafion membrane (top) [64]. Comparison of the MEA performance for the co-PNIS70/30 membrane with pressed and attached electrodes (bottom) [66].
The research on SPNI stability in aqueous environment for several years resulted in the synthesis of a considerable number of novel monomers and polymers. The properties of these materials were investigated and tested under operational conditions of hydrogen–air and methanol–air fuel cells. The current work in this area is being successfully conducted and demonstrates its high practical significance.
Polybenzimidazole-based proton-exchange membranes
One of the PBI types used as phosphoric acid-doped membranes for HT–PEMFC is poly[(1-(4,4'-diphenyl ether)-5-hydroxybenzimidazole)benzimidazole] (PBI-O-O) (Fig. 7) [72, 73].
Figure 7. Chemical structures of PBI-O-O and Py-PBI.
A membrane based on this material and cross-linked with sulfophenylated titanium oxide particles (6 wt % of ps-TiO2), exhibits proton conductivity of 98 mS/cm and phosphoric acid uptake of 392%. At the same time, the MEA reaches a power density of 356 mW/cm2 at 160 °C, which is 76% higher than that for cross-linked PBI-O-O (202 mW/cm2). Meanwhile, the ionically cross-linked membrane (PBI-O-O/sulfonated sulfone) can be converted into a covalently cross-linked one under heating. Its proton conductivity reached 260 mS/cm at 160 °C and a relative humidity of 5% with phosphoric acid uptake of just 266% and the power density maximum of 452 mW/cm2 [72, 73].
The pyridine-PBI (Py-PBI) membranes (Fig. 7) could be excellent examples for HT–PEMFC MEA due to their high proton conductivity. Py-PBIs were obtained from the corresponding tetraamines and pyridinedicarboxylic acids through substitution in the pyridine ring at 2,4-, 2,5-, and 2,6-positions [74]. At the same time, the proton conductivity of the best membranes based on these polymers reaches 200 mS/cm at 160–200 °C for 2,5-Py-PBI, at a phosphoric acid doping level of 20.4 mol per mol of polymer units (approximately 20 molecules of phosphoric acid per each polymer unit). Upon substitution of the pyridine ring at 3,5-positions, the proton conductivity of the 3,5-Py-PBI membrane reaches 279 mS/cm [75]. For this MEA, the potential reaches 660 mV at a current density of 200 mA/cm2. The authors reported stable MEA operation for 2300 h at 200 mA/cm2 when the potential is higher than 0.6 V. Nevertheless, despite reported performance, there are serious concerns regarding the stability of the Py-PBI membrane systems under operational conditions in HT–PEMFC MEA.
One of the most interesting types of membranes is a blend of PBI with partially fluorinated arylene polyethers (FAPE) doped with phosphoric acid (Fig. 8).
Figure 8. Chemical structures of FAPE, PBI-Ph, PBI-O-PhT, and Nu-PBI.
Li et al. [14] produced a three-component blend of sulfonated FAPE with PBI and phosphoric acid. Due to the presence of ionic cross-links which hinder the approach of phosphoric acid to the imidazole centers, doping with highly concentrated phosphoric acid (85%) is necessary up to 130 °C. The ratio of FAPE to PBI in the mixture is 30% FAPE/70% PBI. At a doping level of 6.6 molecules of phosphoric acid per a polymer unit of PBI, the proton conductivity was low and reached only 40 mS/cm at 180 °C. However, when the level of doping was raised to 11 molecules of phosphoric acid per a polymer unit of PBI, the proton conductivity was already 120 mS/cm at 175 °C. The potential values for a single MEA at current densities of 200 and 400 mA/cm2 were 0.62 and 0.54 V, respectively, when using the mixed membrane with a doping degree of 11 phosphoric acid molecules per a polymer unit of PBI. When operating the assembled battery consisting of 44 MEA, a power of 2 kW was recorded with the active surface area of 256 cm2 at 170 °C.
Li et al. [76] suggested imidazole-based cross-linking agents (Fig. 9) with PBI to produce the c-PBI cross-linked membrane.
Figure 9. Chemical structures of imidazole-based cross-linking agent A2B2 (left) and conventional cross-linking agent (right)
for producing the c-PBI membrane.
Unlike the conventional cross-linking agents, which lead to a reduction in the proton conductivity, the imidazole-based cross-linking agent aims at least to maintain the proton conductivity. The main advantage of this cross-linking agent is its ability to react with each other and with the PBI chain and form hydrogen bonds between these molecules, creating a proton-conducting network with high proton conductivity when doped with phosphoric acid. At a cross-linking degree of 30%, the proton conductivity for the phosphoric acid-doped c-PBI membrane reached 198 mS/cm at 160 °C and 253 mS/cm at 200 °C, which is almost 2 times higher than the value for the PBI membrane cross-linked with a conventional cross-linking agent. The performance of this membrane in the hydrogen–oxygen HT–PEMFC MEA is characterized by the maximum power density of 533 mW/cm2 at 160 °C [76].
The same research group [77] also suggested the use of aluminum-substituted mesoporous silica Al-MCM-41 as a proton-conducting material in a PBI composite membrane. Using only 9 wt % of the filler in PBI allows obtaining a membrane with a fairly high proton conductivity of 356 mS/cm at 160 °C, which is 392% higher than that for the conventional PBI membranes. The HT–PEMFC MEA with this membrane shows a current density of 393 mA/cm2 at 0.6 V, and the maximum power density reached 516 mW/cm2 at 150 °C (251 mA/cm2 at 0.6 V and 446 mW/cm2 were obtained under analogous conditions for PBI). The increased proton conductivity and MEA performance are explained by the presence of proton-conducting channels and phosphoric acid reserves in the Al-MCM-41 mesopores.
Seo et al. [78] proposed a porous polyhydroxy-SiO2 as a filler for a cross-inked PBI-Ph membrane (Fig. 9) with an imidazole-enriched cross-linking agent. The addition of SiO2 was carried out to enhance the phosphoric acid retention effect. So that, the best results were achieved for a cross-linking degree of 20% and with 2 wt % of the SiO2 filler in the PBI membrane. The phosphoric acid uptake was recorded to be 329 wt % (23.1 phosphoric acid molecules per a polymer unit), which leads to the membrane proton conductivity values of 199 mS/cm at 160 °C and 244 mS/cm at 200 °C. The membrane was tested in the oxygen–hydrogen HT–PEMFC MEA at 160 °C and was shown to be able to achieve a power density of 497 mW/cm.
The PBI-O-PhT membrane (Fig. 9) was obtained from 3,3',4,4'-tetraaminodiphenyl ether and 4,4'-diphenylphthalide dicarboxylic acid in Eaton's reagent (P2O5/MeSO3H) according to the developed procedure [79]. Polymer films were prepared by casting from 10% N-methylpyrrolidone solutions and heating at 350 °C. The films were subsequently soaked in 2% sulfuric acid solution. The cross-linked films were doped with 77% of H3PO4 at 60 °C for 3 days and stored in 85% H3PO4. The equilibrium absorption of phosphoric acid was 280–330%, as calculated using [(M(doped)–M(dry)]/M(dry) formula.
The PBI-O-PhT membrane is promising for the creation of a new generation of domestic PBI membranes for hydrogen–air HT–PEMFC fuel cells. Thus, this polymer possesses high solubility in organic solvents, as well as high thermal and heat resistance. It has been shown that the films based on the PBI-O-PhT exhibit excellent strength characteristics. Polyelectrolyte complexes formed from the films of PBI-O-PhT and o-phosphoric acid (1:2–4 wt/wt) were applied as FC membranes. The performance of the PBI-O-PhT membrane in the HT–PEMFC MEA with Celtec® P1000 electrodes (BASF, Ludwigshafen am Rhein, Germany) were almost similar to that of the commercial Celtec® membrane (m-PBI). At a current density of 400 and 200 mA/cm2, the voltage values were 0.60 and 0.62 V when the HT–PEMFC MEA were tested at 160 and 180 °C, respectively [80].
Another membrane of potential interest is based on the "nucleophilic" polybenzimidazole (NuPBI), or poly(N-phenylene-benzimidazole) (Fig. 8), which can be obtained through the nucleophilic substitution of bis(benzimidazole) and difluorodiphenylsulfone [81, 82]. Similar membranes have also been studied, where -SO2- or -CH2 groups were used instead of the -O- bridge group or when the bridge group was omitted. The equilibrium absorption of phosphoric acid, calculated for a membrane with the -O- bridge group, was 280%.
Testing of the NuPBI membrane in the HT–PEMFC MEA was carried out with the BASF P1000 electrodes at 160 °C. The hydrogen–air HT–PEMFC MEA in a galvanostatic mode at a current of 400 mA/cm2 after operating for 24 h was characterized by a voltage value of 0.570 V. In this case, the open-circuit voltage was 0.880 V. These data are quite close to the data for operation of the MEA with the commercial Celtec® P1000 (m-PBI) membrane (0.60 V, 400 mA/cm2). In order to increase the affinity of the polymers of a NuPBI type to phosphoric acid and slow down the phosphoric acid leaching process [83], a study on the synthesis of polymers and copolymers based on 1,7-dihydrodiimidazo[4,5-b:4',5'-e]pyridine (Fig. 10) were successfully carried out [84, 85].
Figure 10. Chemical structure of the copolymer of NuPBI and DIP.
For the doped films (phosphoric acid uptake 310–320%), the proton conductivity was studied at different temperatures. The copolymers with 10 and 20% of DIP units exhibited high proton conductivity. The membranes with 10% of DIP demonstrated the highest proton conductivity, reaching 113 mS/cm at 180 °C, which is on a par with the best high-temperature proton-conducting membranes described in the literature.
Recently our research group found that a polybenzimidazole containing methoxy-substituents (PBI-OMe) in the side chain, doped with phosphoric acid, undergo self-phosphorylation (Fig. 11) at operation conditions of H2/air HT–PEMFC [86].
Figure 11. Self-phosphorylation of the PBI-OMe/PBI-OP membrane in o-phosphoric acid at 150–200 °C.
As a result, the proton conductivity of such a membrane increases by an order of magnitude and reaches 100 mS/cm at 180 °C. The performance of the membrane–electrode assembly with the PBI-OMe/PBI-OP membrane and Celtec®-P1000 MEA electrodes exceeds that of the commercial analog with the Celazole® membrane (m-PBI). Thus, the achieved peak power was 680 mW/cm2 at 180 °C. High-molecular weight film-forming pre-polymers based on N1,N5-bis(3-methoxyphenyl)-1,2,4,5-benzenetetramine and [1,10-biphenyl]-4,40-dicarbonyl dichloride were obtained by the polyamidation at room temperature. This two-step synthesis of the new PBI through the stage of the fore-polyamide (pre-polymer) obtaining at room temperature, followed by the thermal heterocyclization, allows moving toward membrane cost reduction and elimination of environmental issues related to the industrial PBI production.
Conclusions
In this review, we tried to combine the missing information from previous years and newly obtained data not included in other reviews. For operating temperatures below 100 °C, the PEMs based on the sulfonated PHAs are used. Particularly, polynaphthoyleneimides with SO3H substituents are of great interest. At higher temperatures (>120 °C), the polybenzimidazole-based PEMs doped with phosphoric acid are among the best ones. The review was not intended to cover all PHA-based membranes which are applied (or could be potentially applied) in PEMFC. The consideration of the presented membranes aimed to highlight important achievements and outline the most significant approaches in the field of PEMFC membrane obtaining, with the ultimate goal of overcoming the current MEA challenges for these types of fuel cells.
For LT–PEMFC, the membranes are showing their effectiveness, and further progress is mainly related to the enhancement of membrane hydrolytic stability and attempts to build fuel cell stacks. For HT–PEMFC, further developments are expected in the field of membrane cross-linking, reinforcement, as well as modification of membranes with inorganic compounds. Another approach is a transition to self-phosphorylating membranes and their adaptation to new types of electrodes.
Acknowledgements
This work was performed with support from Hydrogen Energy Center, Sistema Public Joint Stock Financial Corporation (agreement no. 23-03). Access to electronic scientific resources was provided by INEOS RAS with support from the Ministry of Science and Higher Education of the Russian Federation.
References
- E. Afshari, M. Mosharaf-Dehkordi, H. Rajabian, Energy, 2017, 118, 705–715. DOI: 10.1016/j.energy.2016.10.101
- I. A. Stenina, E. Yu. Safronova, A. V. Levchenko, Yu. A. Dobrovolsky, A. B. Yaroslavtsev, Therm. Eng., 2016, 63, 385–398. DOI: 10.1134/S0040601516060070
- G. Pourcelly, Pet. Chem., 2011, 51, 480–491. DOI: 10.1134/S0965544111070103
- Y.-L. Ma, J. S. Wainright, M. H. Litt, R. F. Savinell, J. Electrochem. Soc., 2004, 151, A8. DOI: 10.1149/1.1630037
- A. Kirubakaran, S. Jain, R. K. Nema, Renewable Sustainable Energy Rev., 2009, 13, 2430–2440. DOI: 10.1016/j.rser.2009.04.004
- K. Scott, A. K. Shukla, Rev. Environ. Sci. Bio/Technol., 2004, 3, 273–280. DOI: 10.1007/s11157-004-6884-z
- M. S. Whittingham, R. F. Savinell, T. Zawodzinski, Chem. Rev., 2004, 104, 4243–4244. DOI: 10.1021/cr020705e
- Y. Yin, O. Yamada, K. Tanaka, K.-I. Okamoto, Polym. J., 2006, 38, 197–219. DOI: 10.1295/polymj.38.197
- V. Neburchilov, J. Martin, H. Wan, J. Zhang, J. Power Sources, 2007, 169, 221–238. DOI: 10.1016/j.jpowsour.2007.03.044
- K. Broka, P. Ekdunge, J. Appl. Electrochem., 1997, 27, 281–289. DOI: 10.1023/A:1018476612810
- E.-B. Cho, D. X. Luu, D. Kim, J. Membr. Sci., 2010, 351, 58–64. DOI: 10.1016/j.memsci.2010.01.028
- T. Xu, J.-J. Woo, S.-J. Seo, S.-H. Moon, J. Membr. Sci., 2008, 325, 209–216. DOI: 10.1016/j.memsci.2008.07.036
- Q. Li, J. O. Jensen, R. F. Savinell, N. J. Bjerrum, Prog. Polym. Sci., 2009, 34, 449–477. DOI: 10.1016/j.progpolymsci.2008.12.003
- Q. Li, J. O. Jensen, C. Pan, V. Bandur, M. S. Nilsson, F. Schönberger, A. Chromik, M. Hein, T. Häring, J. Kerres, N. J. Bjerrum, Fuel Cells, 2008, 8, 188–199. DOI: 10.1002/fuce.200800007
- J. A. Asensio, P. Gómez-Romero, Fuel Cells, 2005, 5, 336–343. DOI: 10.1002/fuce.200400081
- C. Wannek, W. Lehnert, J. Mergel, J. Power Sources, 2009, 192, 258–266. DOI: 10.1016/j.jpowsour.2009.03.051
- C. Marestin, G. Gebel, O. Diat, R. Mercier, in: Fuel Cells II, G. G. Scherer (Ed.), Adv. Polym. Sci., Berlin, Heidelberg, Springer, 2008, vol. 216, pp. 185–258. DOI: 10.1007/12_2008_155
- S. V. Vinogradova, V. A. Vasnev, Polycondensation Processes and Polymers, Moscow, Nauka, 2000 (in Russian).
- V. V. Korshak, S. V. Vinogradova, Ya. S. Vygodskii, J. Macromol. Sci., Part C, 1974, 11, 45–142. DOI: 10.1080/15583727408546022
- V. V. Korsak, S. V. Vinogradova, J. S. Vygodskij, Faserforsch. Textiltech., 1977, 28, 439–443.
- I. I. Ponomarev, O. G. Nikolsky, A. L. Rusanov, S. V. Vinogradova, E. S. Obolonkova, N. G. Matvelashvili, V. Yu. Levin, Vysokomol. Soedin., Ser. B, 1990, 32, 637–638.
- I. I. Ponomarev, O. G. Nikolsky, in: Polyimides and Other High-Temperature Polymers, M. J. M. Abadie, B. Sillion (Eds.) Amsterdam, Elsevier, 1991, pp. 207–214.
- O. G. Nikol'skii, I. I. Ponomarev, N. S. Perov, V. A. Martirosov, Acoust. Phys., 2003, 49, 704–710. DOI: 10.1134/1.1626184
- V. V. Lyapunov, E. N. Lyakh, V. A. Solomin, B. A. Zhubanov, Dokl. Akad. Nauk, 1992, 326, 106–108.
- I. I. Ponomarev, O. G. Nikolsky, Y. A. Volkova, A. V. Zakharov, Vysokomol. Soedin., 1994, 36, 1429–1437.
- G. I. Timofeeva, I. I. Ponomarev, A. R. Khokhlov, R. Mercier, B. Sillion, Macromol. Symp., 1996, 106, 345–351. DOI: 10.1002/masy.19961060132
- H. Ahmad, S. K. Kamarudin, U. A. Hasran, W. R. W. Daud, Int. J. Hydrogen Energy, 2010, 35, 2160–2175. DOI: 10.1016/j.ijhydene.2009.12.054
- F. Lufrano, V. Baglio, P. Staiti, V. Antonucci, A. S. Arico', J. Power Sources, 2013, 243, 519–534. DOI: 10.1016/j.jpowsour.2013.05.180
- S. Faure, N. Cornet, G. Gebel, R. Mercier, M. Pineri, B. Sillion, Proc. Second Int. Symp. on New Materials for Fuel Cells and Modern Battery Systems, Montreal, Canada, 1997, pp. 818–827.
- Y. Zhang, M. Litt, R.F. Savinell, J.S. Wainrigth, Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.), 1999, 40, 480–481.
- N. Cornet, O. Diat, G. Gebel, F. Jousse, D. Marsacq, R. Mercier, M. Pineri, J. New Mat. Electrochem. Systems, 2000, 3, 33–42.
- S. Besse, P. Capron, O. Diat, G. Gebel, F. Jousse, D. Marsacq, M. Pineri, C. Marestin, R. Mercier, J. New Mater. Electrochem. Syst., 2002, 5, 109–112.
- X. Guo, J. Fang, T. Watari, K. Tanaka, H. Kita, K.-i. Okamoto, Macromolecules, 2002, 35, 6707–6713. DOI: 10.1021/ma020260w
- J. F. Blachot, O. Diat, J.-L. Putaux, A.-L. Rollet, L. Rubatat, C. Vallois, M. Muller, G. Gebel, J. Membr. Sci., 2003, 214, 31–42. DOI: 10.1016/S0376-7388(02)00522-7
- Y. Yin, J. Fang, Y. Cui, K. Tanaka, H. Kita, K.-i. Okamoto, Polymer, 2003, 44, 4509–4518. DOI: 10.1016/S0032-3861(03)00439-7
- N. Asano, K. Miyatake, M. Watanabe, Chem. Mater., 2004, 16, 2841–2843. DOI: 10.1021/cm049550c
- G. Meyer, G. Gebel, L. Gonon, P. Capron, D. Marscaq, C. Marestin, R. Mercier, J. Power Sources, 2006, 157, 293–301. DOI: 10.1016/j.jpowsour.2005.07.049
- R. S. Barshtein, I. A. Sorokina, Catalytic Polycondensation, Moscow, Khimiya, 1988 (in Russian).
- I. I. Ponomarev, Dr. Sci. Dissertation, Moscow, INEOS RAS, 1991.
- E. G. Bulycheva, M. P. Prigozhina, I. I. Ponomarev, B. A. Butskhrikide, R. P. Tsiskarishvili, A. L. Rusanov, S. V. Vinogradova, Z. Jedlinski, A. Palivoda, Acta Polym., 1991, 42, 63–66. DOI: 10.1002/actp.1991.010420203
- X. Chen, Y. Yin, P. Chen, H. Kita, K.-I. Okamoto, J. Membr. Sci., 2008, 313, 106–119. DOI: 10.1016/j.memsci.2007.12.069
- K. Miyatake, H. Zhou, H. Uchida, M. Watanabe, Chem. Commun., 2003, 368–369. DOI: 10.1039/B210296J
- K. Miyatake, N. Asano, M. Watanabe, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 3901–3907. DOI: 10.1002/pola.10988
- K. Miyatake, H. Zhou, T. Matsuo, H. Uchida, M. Watanabe, Macromolecules, 2004, 37, 4961–4966. DOI: 10.1021/ma049547e
- K. Miyatake, H. Zhou, M. Watanabe, Macromolecules, 2004, 37, 4956–4960. DOI: 10.1021/ma0495487
- C. Lee, S. Sundar, J. Kwon, H. Han, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 3612–3620. DOI: 10.1002/pola.20214
- G. Gebel, L. Gonon, G. Meyer, C. Perrot, Proc. 15th Annual Meet. of NAMS, Honolulu, USA, 2004.
- C. Perrot, L. Gonon, C. Marestin, G. Gebel, J. Membr. Sci., 2011, 379, 207–214. DOI: 10.1016/j.memsci.2011.05.063
- Y. Yin, O. Yamada, K. Tanaka, K.-I. Okamoto, Polymer J., 2006, 38, 197–219. DOI: 10.1295/polymj.38.197
- Y. Yin, S. Chen, X. Guo, G. Fang, K. Tanaka, X. Kita, K.-I. Okamoto, High Perform. Polym., 2006, 18, 617–635. DOI: 10.1177/0954008306068224
- K. Miyatake, T. Yasuda, M. Watanabe, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 4469–4478. DOI: 10.1002/pola.22782
- H. Han, C. C. Gryte, M. Ree, Polymer, 1995, 36, 1663–1672. DOI: 10.1016/0032-3861(95)99012-J
- J. Seo, K.-Y. Cho, H. Han, Polym. Degrad. Stab., 2001, 74, 133–137. DOI: 10.1016/S0141-3910(01)00113-6
- M. S. Levenets, Cand. Sci. Dissertation, Moscow, IPC RAS, 1996.
- F. Piroux, E. Espuche, R. Mercier, M. Pinéri, J. Membr. Sci., 2003, 223, 127–139. DOI: 10.1016/S0376-7388(03)00315-6
- X. Guo, J. Fang, K. Tanaka, H. Kita, K.-i. Okamoto, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 1432–1440. DOI: 10.1002/pola.11063
- A. G. Kumar, D. Bera, S. Banerjee, R. Veerubhotla, D. Das, Eur. Polym. J., 2016, 83, 114–128. DOI: 10.1016/j.eurpolymj.2016.08.009
- A. Ganeshkumar, D. Bera, E. A. Mistri, S. Banerjee, Eur. Polym. J., 2014, 60, 235–246. DOI: 10.1016/j.eurpolymj.2014.09.009
- A. K. Mandal, D. Bera, S. Banerjee, Mater. Chem. Phys., 2016, 181, 265–276. DOI: 10.1016/j.matchemphys.2016.06.058
- Z. Wu, S. Zhang, H. Li, Y. Liang, Z. Qi, Y. Xu, Y. Tang, C. Gong, J. Power Sources, 2015, 290, 42–52. DOI: 10.1016/j.jpowsour.2015.04.174
- X. Zhu, H. Pan, Y. Liang, X. Jian, Eur. Polym. J., 2008, 44, 3782–3789. DOI: 10.1016/j.eurpolymj.2008.08.018
- N. Asano, M. Aoki, S. Suzuki, K. Miyatake, H. Uchida, M. Watanabe, J. Am. Chem. Soc., 2006, 128, 1762–1769. DOI: 10.1021/ja0571491
- I. I. Ponomarev, V. A. Grinberg, V. V. Emets, N. A. Maiorova, M. Yu. Zharinova, Yu. A. Volkova, D. Yu. Razorenov, K. M. Skupov, E. A. Nizhnikovskii, Russ. J. Electrochem., 2016, 52, 525–532. DOI: 10.1134/S1023193516060100
- U. M. Zavorotnaya, I. I. Ponomarev, Y. A. Volkova, V. V. Sinitsyn, Membranes, 2023, 13, 485. DOI: 10.3390/membranes13050485
- V. V. Emets, I. I. Ponomarev, V. A. Grinberg, N. A. Mayorova, M. Yu. Zharinova, Yu. A. Volkova, E. A. Nizhnikovskii, K. M. Skupov, D. Yu. Razorenov, V. N. Andreev, Iv. I. Ponomarev, Russ. J. Electrochem., 2017, 53, 86–91. DOI: 10.1134/S1023193517010062
- U. M. Zavorotnaya, I. I. Ponomarev, Y. A. Volkova, A. D. Modestov, V. N. Andreev, A. F. Privalov, M. Vogel, V. V. Sinitsyn, Materials, 2020, 13, 5297. DOI: 10.3390/ma13225297
- U. M. Zavorotnaya, A. F. Privalov, B. Kresse, M. Vogel, I. I. Ponomarev, Y. A. Volkova, V. V. Sinitsyn, Macromolecules, 2022, 55, 8823–8833. DOI: 10.1021/acs.macromol.2c01486
- J. Saito, K. Miyatake, M. Watanabe, Macromolecules, 2008, 41, 2415–2420. DOI: 10.1021/ma7028055
- A. Kabasawa, J. Saito, H. Yano, K. Miyatake, H. Uchida, M. Watanabe, Electrochim. Acta, 2009, 54, 1076–1082. DOI: 10.1016/j.electacta.2008.08.042
- H. Yao, Y. Zhang, Y. Liu, K. You, N. Song, B. Liu, S. Guan, J. Membr. Sci., 2015, 480, 83–92. DOI: 10.1016/j.memsci.2014.12.014
- H. Yao, K. Shi, N. Song, N. Zhang, P. Huo, S. Zhu, Y. Zhang, S. Guan, Polymer, 2016, 103, 171–179. DOI: 10.1016/j.polymer.2016.09.049
- N. N. Krishnan, D. Joseph, N. M. H. Duong, A. Konovalova, J. H. Jang, H.-J. Kim, S. W. Nam, D. Henkensmeier, J. Membr. Sci., 2017, 544, 416–424. DOI: 10.1016/j.memsci.2017.09.049
- N. N. Krishnan, S. Lee, R. V. Ghorpade, A. Konovalova, J. H. Jang, H.-J. Kim, J. Han, D. Henkensmeier, H. Han, J. Membr. Sci., 2018, 560, 11–20. DOI: 10.1016/j.memsci.2018.05.006
- L. Xiao, H. Zhang, T. Jana, E. Scanlon, R. Chen, E.-W. Choe, L. S. Ramanathan, S. Yu, B. C. Benicewicz, Fuel Cells, 2005, 5, 287–295. DOI: 10.1002/fuce.200400067
- M. A. Molleo, X. Chen, H. J. Ploehn, K. J. Fishel, B. C. Benicewicz, Fuel Cells, 2014, 14, 16–25. DOI: 10.1002/fuce.201300202
- X. Li, H. Ma, P. Wang, Z. Liu, J. Peng, W. Hu, Z. Jiang, B. Liu, M. D. Guiver, Chem. Mater., 2020, 32, 1182–1191. DOI: 10.1021/acs.chemmater.9b04321
- X. Li, H. Ma, P. Wang, Z. Liu, J. Peng, W. Hu, Z. Jiang, B. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 30735–30746. DOI: 10.1021/acsami.9b06808
- K. Seo, K.-H. Nam, H. Han, Sci. Rep., 2020, 10, 10352. DOI: 10.1038/s41598-020-66935-5
- RU Patent 2332429, 2008.
- M. S. Kondratenko, I. I. Ponomarev, M. O. Gallyamov, D. Y. Razorenov, Y. A. Volkova, E. P. Kharitonova, A. R. Khokhlov, Beilstein J. Nanotechnol., 2013, 4, 481–492. DOI: 10.3762/bjnano.4.57
- I. I. Ponomarev, I. I. Ponomarev, Y. A. Volkova, M. Yu. Zharinova, D. Yu. Razorenov, Mendeleev Commun., 2012, 22, 162–163. DOI: 10.1016/j.mencom.2012.05.018
- D. Y. Razorenov, K. M. Skupov, Y. A. Volkova, I. I. Ponomarev, E. M. Chaika, M. I. Buzin, I. V. Blagodatskikh, I. I. Ponomarev, Macromol. Symp., 2017, 375, 1600152. DOI: 10.1002/masy.201600152
- F. Gao, X. Li, X. Zhang, W. Liu, C. Liu, Colloids Surf., A, 2020, 603, 125197. DOI: 10.1016/j.colsurfa.2020.125197
- D. Yu. Razorenov, S. A. Makulova, I. V. Fedyanin, K. A. Lyssenko, K. M. Skupov, Yu. A. Volkova, Iv. I. Ponomarev, I. I. Ponomarev, Mendeleev Commun., 2019, 29, 181–183. DOI: 10.1016/j.mencom.2019.03.022
- I. I. Ponomarev, D. Yy. Razorenov, V. A. Muravyova, K. M. Skupov, Yu. A. Volkova, Iv. I. Ponomarev, M. M. Ilyin, E. M. Chaika, Polym. Sci., Ser. C, 2020, 62, 214–221. DOI: 10.1134/S1811238220020101
- I. I. Ponomarev, D. Y. Razorenov, K. M. Skupov, Iv. I. Ponomarev, Y. A. Volkova, K. A. Lyssenko, A. A. Lysova, E. S. Vtyurina, M. I. Buzin, Z. S. Klemenkova, Membranes, 2023, 13, 552. DOI: 10.3390/membranes13060552





