2023 Volume 6 Issue 3 (Published 30 June 2024)
|
INEOS OPEN, 2023, 6 (3), 86–90 Journal of Nesmeyanov Institute of Organoelement Compounds |
|
Methyldiphenylsiloxane MQ Nanogels as Viscosity Regulators
for Liquid Sealing Compositions
A. S. Kholkina,с Yu. P. Zaikov,с and I. Yu. Dalyaev d
a Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, str. 1, Moscow, 119334 Russia
b Enikolopov Institute of Synthetic Polymeric Materials, Russian Academy of Sciences, ul. Profsoyuznaya 70, Moscow, 117393 Russia
с Institute of High Temperature Electrochemistry, Ural Branch of the Russian Academy of Sciences, ul. Akademicheskaya 20, Yekaterinburg, Sverdlovsk Oblast, 620066 Russia
d Central Research and Development Institute of Robotics and Technical Cybernetics, Tikhoretskii pr. 21, St. Petersburg, 194064 Russia
Corresponding author: N. G. Mazhorova, e-mail: ngmazhorova@mail.ru
Received 7 December 2023; accepted 15 February 2024
Abstract
A new phenyl-containing MQ nanogel was obtained by the polycondensation of tetraethoxysilane and methyldiphenylethoxysilane in glacial acetic acid and characterized. Its effectiveness as a molecular thickener for siloxane oils was demonstrated. It was shown that its introduction into a poly(methylphenylsiloxane) liquid leads to an increase in the viscosity while maintaining the thermal and sealing characteristics.
Key words: phenyl-containing MQ nanogels, poly(methylphenylsiloxane), molecular thickener, siloxane-based grease.
Introduction
Greases are complex multicomponent compositions, one of the main components of which, besides an oil base, is a thickener [1–4]. Its role is to regulate the viscosity of the composition and ensure thixotropic properties, namely, a transition of lubricants from a plastic (pseudo-solid) to viscous-flow (liquid) state under shear forces that exceed the tensile strength of the grease, and vice versa. For organic lubricants, the most common thickeners are soaps: salts of fatty acids with alkali or alkaline earth metals [5, 6]. Such thickeners are also frequently used in the production of siloxane greases, but their efficiency is significantly lower, and therefore they are usually used along with polymeric thickeners, for example, aromatic polyureas [7–9]. This significantly reduces the operating range of siloxane greases, which is one of their main advantages.
Siloxane oils, which serve as the basis for creating compositions, are represented by a quite large range of products and are widely used for the production of lubricants operating under extreme conditions. Unlike mineral and most synthetic oils, the drawbacks of which are low viscosity-temperature characteristics (viscosity index 30–40), as well as insufficient thermal and thermooxidative stability, siloxane oils show a low viscosity dependence on temperature (viscosity index 150–300), are operable at 150–250 °C, and the high content of phenyl groups in the composition endow siloxane oils with resistance to prolonged heating at temperatures above 300 °C and resistance to radiation (1 MGy) [10–14]. In this respect, poly(methylphenylsiloxane) (PMPS) liquids have an advantage over poly(dimethylsiloxane) counterparts.
Therefore, from this point of view, the creation of thickeners that can ensure control over the system viscosity without deteriorating the main thermal characteristics is an urgent task. Recent investigations [15], including our group research [16–20], have shown that the use of various siloxane nanogels in the compositions based on liquid siloxane rubbers allows for considering them as molecular fillers and provides effective control over the properties of such compositions.
Densely cross-linked nanogels (in particular, MQ resins used in the present work) are polycyclic network spatial structures, the sizes of which are artificially limited using different methods: either by considerable dilution in the gas phase or in an organic solvent, or by adding a blocking agent that terminates the particle growth [21].
The dual nature of macromolecule-particles allows, on the one hand, for considering them as promising fillers, and on the other hand, fine-tuning the properties of the resulting compositions, which only increases their potential as molecular fillers [15, 22–24]. The rheological properties, such as elastic modulus, viscosity, and strength, which characterize such systems (dispersion medium and disperse phase), depend on the chemical nature of nanoparticles, their size, concentration, compatibility with a basic oil, and their stabilization in lubricants [25–33].
As is known, phenyl-containing MQ resins have good compatibility with phenyl-containing silicone liquids and offer advantages such as high refractive index, radiation resistance, and stability at high and low temperatures [34].
Hence, the goal of this work was to obtain new methyl phenyl MQ nanogels and study their properties in lubricating compositions based on model PMPS liquids.
Results and discussion
The methyl phenyl nanogel in use was an MQ resin obtained by the polycondensation of methyldiphenylethoxysilane and tetraethoxysilane, taken in a 1:1 ratio, in an active medium, namely, anhydrous acetic acid, according to the method developed by our research group (Scheme 1) [35, 36].
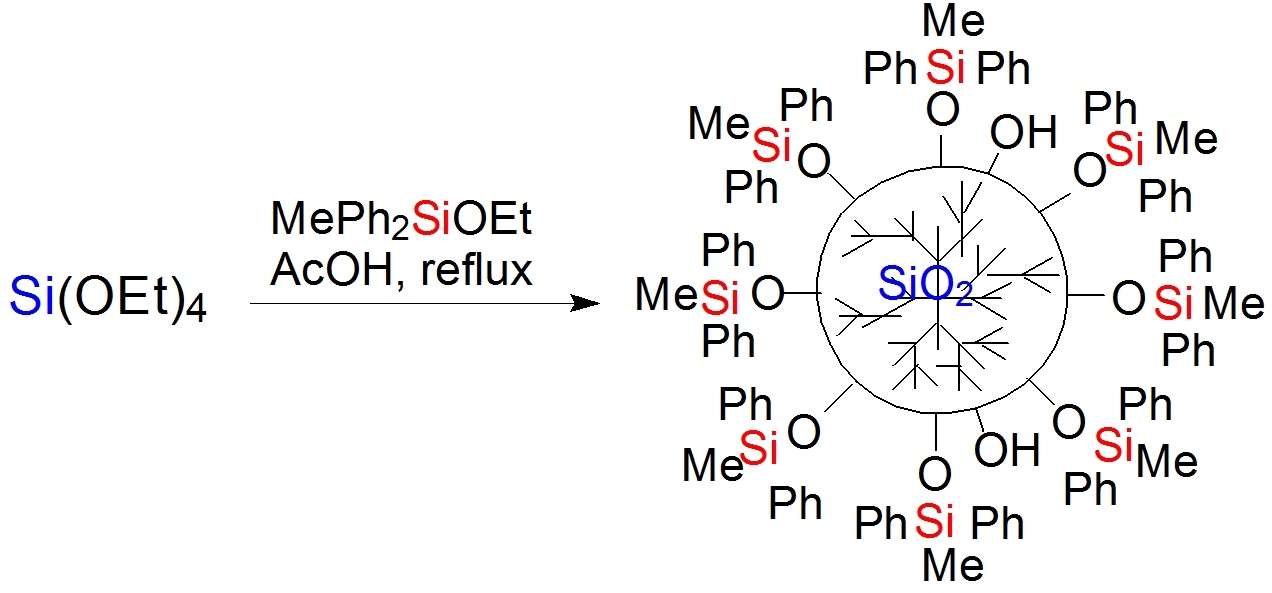
Scheme 1. Polycondensation of tetraethoxysilane and methyldiphenylethoxysilane in glacial acetic acid.
A mixture of methyldiphenylethoxysilane and tetraethoxysilane was refluxed in acetic acid until complete conversion of alkoxy groups, which was determined by the intensity of signals of methyl and methylene protons of the ethoxy groups at ca. 1.5 and 3.5 ppm, respectively, in the 1H NMR spectra of the samples, taken from the reaction mixture, after removal of volatile products. The 1H NMR spectrum of the sample taken in 14 h after the reaction beginning did not reveal the signals of the ethoxy groups, which indicated their complete conversion (Fig. 1).
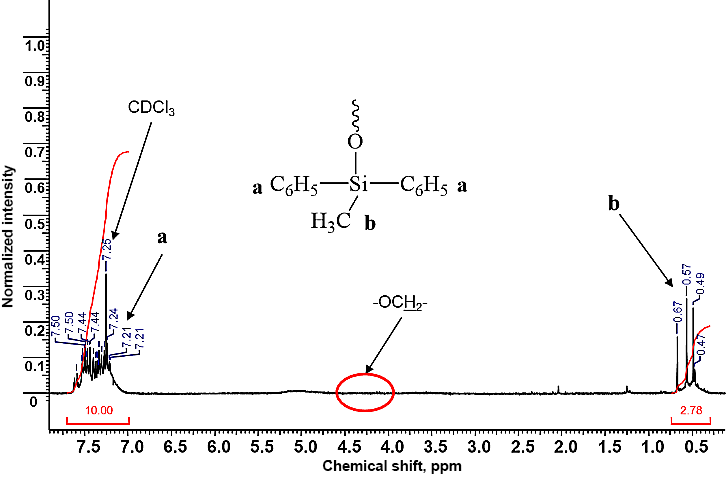
Figure 1. 1H NMR spectrum of the product of the polycondensation of methyldiphenylethoxysilane
and tetraethoxysilane in acetic acid obtained in 14 h (CDCl3).
The ratio of the integral intensities of the signals of methyl and phenyl groups at 0.47–0.67 and 7.21–7.50 ppm corresponded to those calculated for methyldiphenylsiloxy units.
A crude product was obtained in 99% yield. According to the results of gel permeation chromatography (GPC) analysis (Fig. 2a), the product featured a polymodal molecular weight distribution and contained 25% of low molecular weight components, presumably consisting of dimethyltetraphenyldisiloxane and methyldiphenylsilanol.
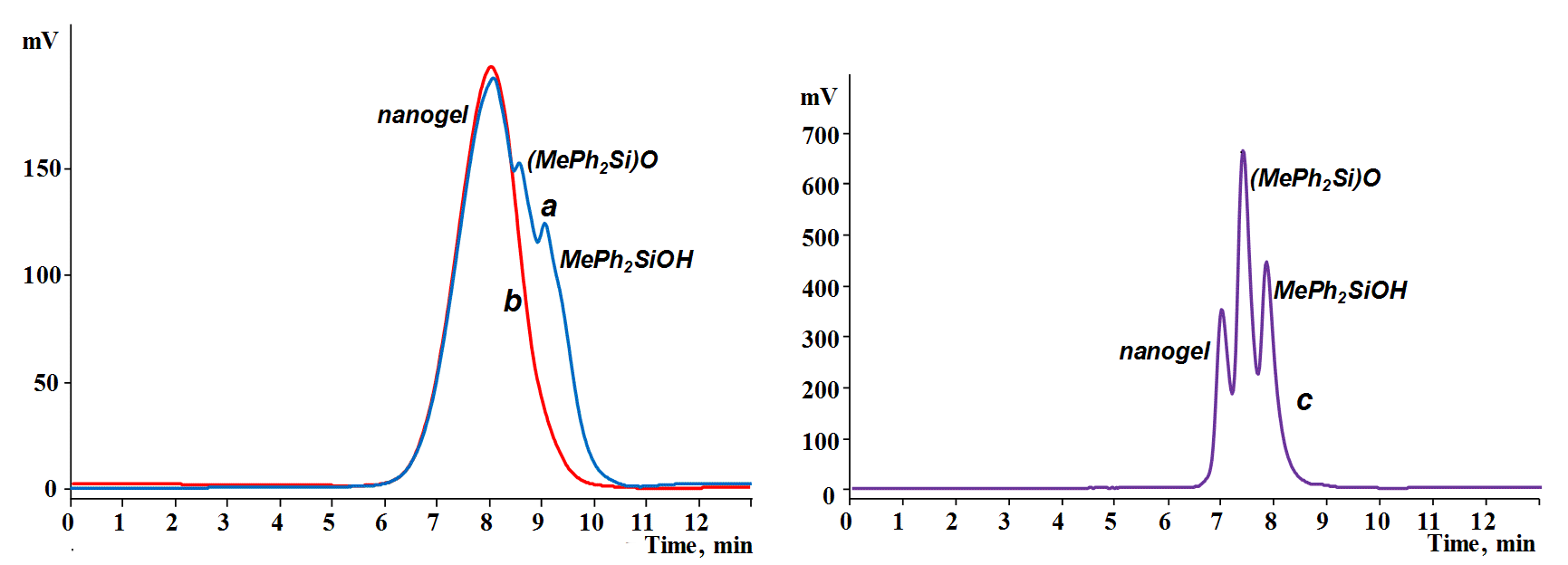
Figure 2. GPC curves of the phenyl-containing MQ nanogel before (a, THF, 40 kDa) and after distillation of
volatile products (b, THF, 40 kDa), as well as that of the distillate (c, THF, 20 kDa).
To obtain the target MQ nanogel deprived of the low molecular weight impurities, the crude sample was distilled under vacuum to give the neat product in 74% yield as a light-yellow glassy solid resin.
Figure 2b shows the GPC curve of the target phenyl-containing MQ nanogel. The product was characterized by a monomodal distribution with Mw/Mn = 1.64, Mn = 1700, and Mw = 2700.
The analysis of the distilled fraction, performed using dimethyltetraphenyldisiloxane and methyldiphenylsilanol as standards, allowed us to establish the following composition: 45% of dimethyltetraphenyldisiloxane, 33% of methyldiphenylsilanol, and 22% of the low molecular weight part of the nanogel (Fig. 2c). These data suggest that 62% of starting methyldiphenylethoxysilane was included in the nanogel and 38% of it was converted to dimethyltetraphenyldisiloxane and methyldiphenylsilanol, i.e., the actual ratio of the M and Q units in the resulting nanogel is 1 to 1.61.
Figure 3 (green spectrum) shows the 1H NMR spectrum of the resulting product. As can be seen, it contains more broadened signals of protons of the methyl and phenyl substituents at the silicon atom than the spectrum of the polycondensation product depicted in Fig. 1.
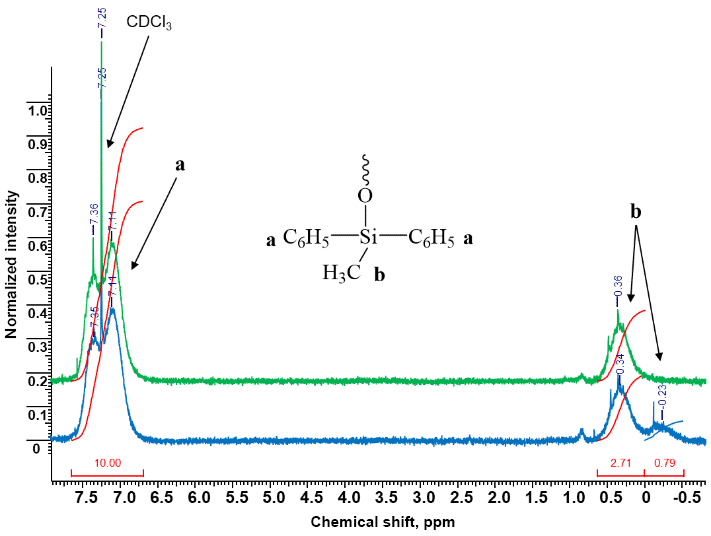
Figure 3. 1H NMR spectra of the target phenyl-containing MQ nanogel after steam distillation at 300 °C (green)
and its trimethylsilylated analog (blue) (CDCl3).
To determine the content of residual silanol groups in the phenyl-containing MQ nanogel, a blocking procedure was carried out according to the earlier described method (Scheme 2) [35].
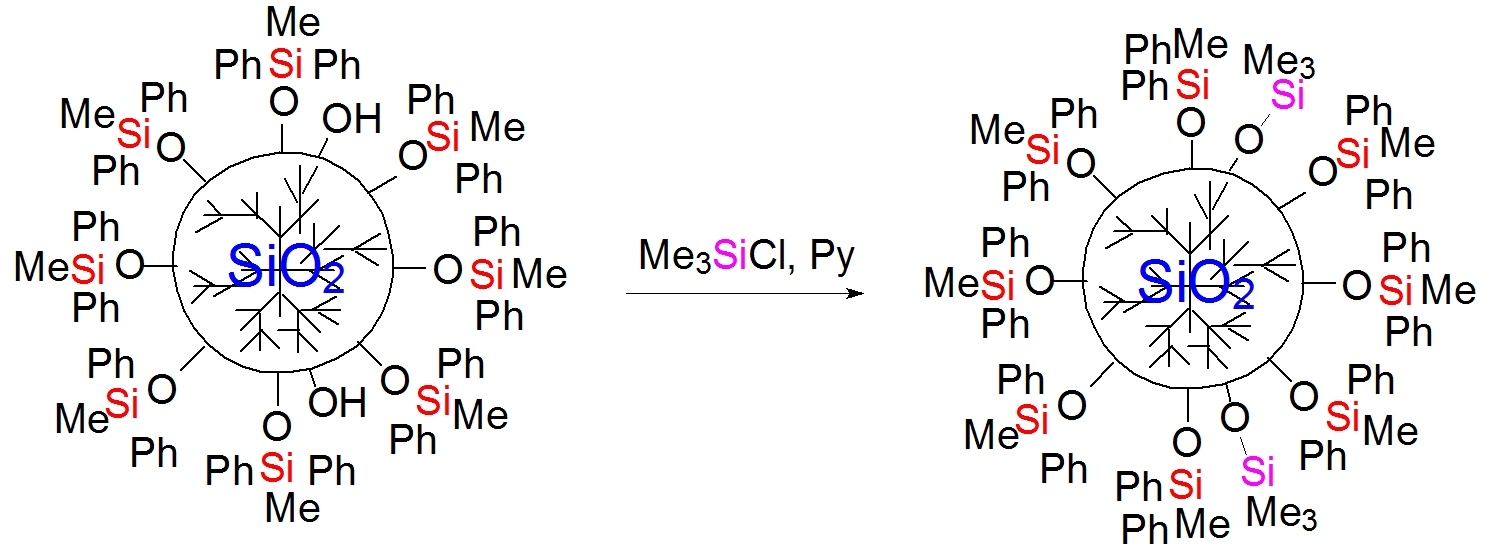
Scheme 2. Trimethylsilylation of the phenyl-containing MQ nanogel.
Based on an increase in the total integral intensity of the signals of protons of the methyl groups at the silicon atom at 0.36–0.49 ppm in the 1H NMR spectra of the phenyl-containing MQ nanogel after trimethylsilylation (Fig. 3, blue) compared to the spectrum of the initial sample (Fig. 3, green), the content of the silanol groups in the phenyl-containing MQ nanogel was found to be 0.6 wt %.
Thus, the polycondensation of tetraethoxysilane and methyldiphenylethoxysilane in acetic acid afforded the phenyl-containing MQ-nanogel with Mw/Mn = 1.64, Mn = 1700, Mw = 2700, and 0.6 wt % of residual hydroxy groups in 74% yield. The nanogel is soluble at room temperature in most polar (for example, tetrahydrofuran, acetone, methyl tert-butyl ether (MTBE)) and non-polar (for example, toluene, benzene) organic solvents. The ratio of the M and Q units according to the elemental analysis data was 1/1.60, which is in good agreement with the data calculated from the 1H NMR spectrum: 1/1.61.
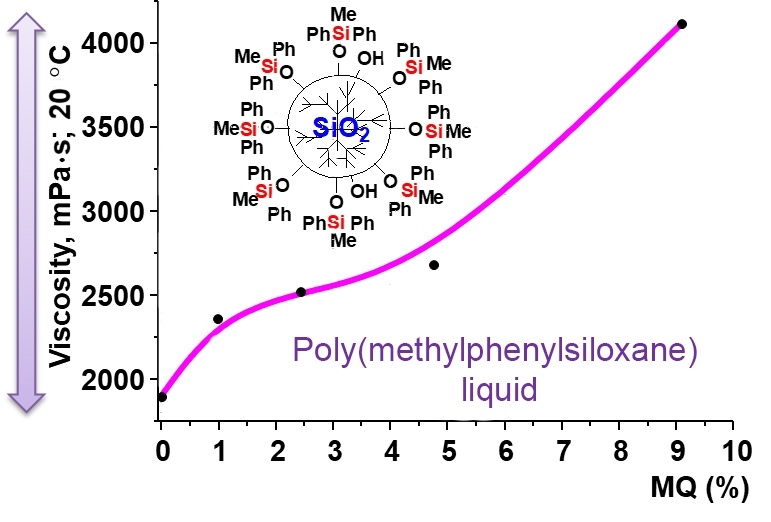
A PMPS liquid bearing methyldiphenylsiloxy terminal units was used as a basis for producing the sealing compositions of various viscosities and evaluation of thickening ability of the resulting phenyl-containing MQ nanogel. The compositions based on it with the addition of the phenyl-containing MQ nanogel were low-viscosity transparent liquids (the transparency was determined visually in transmitted light). The liquids remained transparent for a long period of time when stored under normal conditions, indicating good compatibility between two system components (Fig. 4).
 |
 |
|
a
|
b
|
Figure 4. Appearance of the freshly prepared sealing composition based on the PMPS liquid (the MQ nanogel content in the composition
was 0.8 wt %) (a) and that of the same composition after its storage for four months under normal conditions (b).
The formulations and characteristics of the resulting compositions are listed in Table 1.
Table 1. Formulations and characteristics of the sealing compositions based on the PMPS liquid and phenyl-containing MQ nanogel
|
С, wt %a
|
|
μ, ±10 mPa·s
|
σ·103, ± 0.5 N/m
|
Θ, ± 0.2 deg
|
|
|
Steel
|
|||||
|
12Х18Н10Т
|
UPSt
|
||||
|
0
|
1.5439
|
1850
|
25.5
|
24.8
|
24.7
|
|
0.8
|
1.5455
|
2310
|
25.6
|
24.6
|
24.5
|
|
4.8
|
1.5471
|
2580
|
25.6
|
23.0
|
21.2
|
|
9.1
|
1.5480
|
4070
|
26.4
|
22.2
|
21.0
|
|
a mass fraction of the phenyl-containing MQ nanogel in the composition;
σ is the coefficient of surface tension of the composition at the interface with air at 25 °C;
Θ is the contact angle of steel surfaces at 25 °C: stainless steel 12Х18Н10Т and carbon spring steel UPSt;
μ is the dynamic viscosity of the composition at 20 °C;
|
The resulting coefficient of surface tension (σ) of the neat PMPS liquid at the interface with air at 25 °C was no less than 25 mN/m. In this case, the introduction of different amounts of the phenyl-containing MQ nanogel into the PMPS liquid does not affect the value of σ of the resulting composition, which ranges within 25–27 mN/m.
The contact angles of the resulting compositions were determined on the surfaces of two steel grades: stainless steel 12Х18Н10Т and carbon spring steel UPSt. The introduction of the phenyl-containing MQ nanogel into the PMPS liquid, even in small concentrations (no more than 5%), appeared to lead to a decrease in the contact angle from 24.8 to 23.0 deg in the case of 12Х18Н10Т steel and from 24.7 to 21.2 deg for UPSt steel and, accordingly, an increase in the sealing properties of the compositions.
The refractive index of the compositions increased monotonically with increasing degree of PMPS filling with the MQ nanogel.
The values of weight loss in argon at 350 °C for the compositions obtained and for the PMPS liquid were comparable and did not exceed 5 wt %, which indicates their high thermal stability.
The DSC curves of the PMPS–MQ compositions exhibited only one temperature transition, which testifies the molecular level of filling. Figure 5 shows an example of such a dependence.
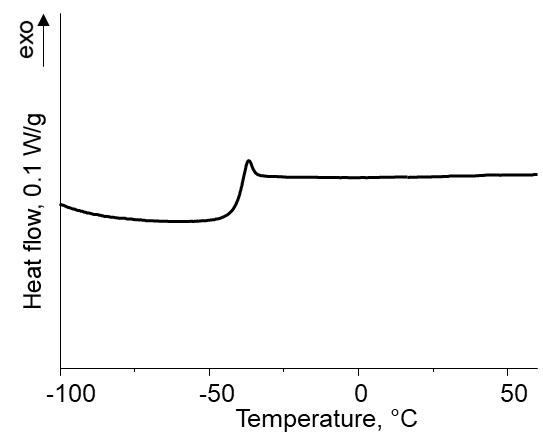
Figure 5. Dependence of the heat capacity on temperature during DSC measurements of the PMPS composition bearing 0.8% of the MQ nanogel.
Hence, the research into the properties of the compositions based on the phenyl-containing MQ nanogel obtained and PMPS liquid showed that the use of the nanogel as a molecular filler ensures effective control over the viscosity of the resulting compositions while maintaining the thermal characteristics and improving the surfactant properties.
Experimental section
Materials
Tetraethoxysilane, toluene, MTBE, acetic acid, pyridine of reagent grade (Komponent Reaktiv, Russia), trimethylchlorosilane, and methyldiphenylethoxysilane (Aladdin, China) were purchased from commercial sources and used without purification. The PMPS liquid bearing terminal methyldiphenylsiloxy units and featuring the viscosity of 1850 mPa·s and the molecular weight characteristics Mw = 3400, Mn = 2070, Mw/Mn = 1.64 was used as a basis for obtaining the sealing compositions of various viscosities and assessing the thickening ability of the resulting phenyl-containing MQ nanogel.
Methods
The 1H NMR spectra were recorded on a Bruker WP-250 SY instrument at an operating frequency of 250 MHz. The chemical shifts were determined relative to deuterochloroform (δ = 7.25 ppm). The spectra were processed using the ACDLABS software package.
The analytical GPC studies were performed on a chromatographic system consisting of a Styer series 2 high-pressure pump (Akvilon, Russia), a RIDK 102 refractive index detector (Czech Republic), a JETSTREAM 2 PLUS column thermostat (KNAUER, Germany), columns with a length of 300 mm and a diameter of 7.8 mm, filled with Phenogel sorbent (Phenomenex, USA) with a particle size of 5 mm and a pore size of 103A and 104A (with a separation range of up to 75000 D and 500000 D, respectively). THF was used as an eluent at a flow rate of 1.0 mL/min. The data were registered and processed using the UniChrom 4.7 program (Belarus), assigning the molecular weights relative to polystyrene standards.
The thermogravimetric analysis was carried out on a Shimadzu DTG-60H synchronous thermal analyzer (Japan) with simultaneous recording of TG and DTA signals in the temperature range of 30–700 °C at a heating rate of 10 °C/min in an argon atmosphere.
The DSC measurements were conducted on a DSC3 instrument (Mettler Toledo, Switzerland) at a heating rate of 10 °C/min.
The viscosities of the compositions were determined on a Brookfield Haake Vicostester 7 plus rotational viscometer (Thermo Scientific, Germany). The measurements were carried out at a temperature of 20 ± 0.5 °C using an L4 spindle at a spindle rotation speed of 60 rpm.
The refractive index was measured using an IRF-22 refractometer (USSR) at 25 °C.
The surfactant properties of the compositions were determined by strain measurement using an EasyDrop DSA1 unit (KRÜSS GmbH, Hamburg, Germany). The coefficient of surface tension of the compositions at the interface with air at 25 °C was calculated using the hanging drop method. The contact angles of the resulting compositions were determined on the surfaces of two steel grades :12Х18Н10Т and UPSt.
The carbon and hydrogen elemental analyses were carried out by express gravimetric analysis. The method is based on the pyrolytic combustion of a 3–5 mg sample of the substance in a platinum boat in a quartz tube in an oxygen flow at 1000 °C in the presence of PbO. The sample combustion results in the quantitative formation of CO2 and H2O which are absorbed outside the combustion tube by ascarite and anhydrone, respectively. The C and H contents were calculated based on the weight gain of the absorption apparatus. The method error is 0.30–0.50%. The silicon content was determined spectrophotometrically on a Cary-100 instrument in the form of a silicon molybdenum blue complex. The method error is 0.30–0.50%.
Syntheses
Synthesis of the phenyl-containing MQ nanogel. A mixture of methyldiphenylethoxysilane (91.4 g, 0.38 mol), tetraethoxysilane (78.5 g, 0.38 mol), and acetic acid (396.0 g, 6.60 mol) was refluxed at 125 °C over an oil bath for 14 h until the complete disappearance of the signals of alkoxy groups in the 1H NMR spectrum. Then the volatile compounds were removed under vacuum; residual acetic acid was removed by a double azeotropic distillation with toluene. The low-molecular impurities were removed by vacuum distillation upon heating the vapors to 290–300 °C. The target product was obtained as a glassy solid. Yield: 74.0 g (74%). 1H NMR (300 MHz, CDCl3, 298 K): δ 7.36, 7.12 (10H, m, Ph); 0.36 (2.8H, m, Me) ppm. GPC: Mn = 1700, Mw = 2700, Mn/Mw = 1.6. Anal. Calcd: C, 51.7; H, 4.3; Si, 24.2. Found: C, 51.9; H, 4.3; Si, 23.8%.
Blocking of the silanol groups in the MQ nanogel. A sample of the resin (1 g) was dissolved in toluene (25 mL). Then trimethylchlorosilane (1.5 mL) and pyridine (0.95 mL) were added. The reaction mixture was refluxed for 2 h and then washed with water until the neutral pH. The organic layer was dried over anhydrous Na2SO4; the volatile compounds were removed under vacuum at 1 mm Hg.
Production of the compositions. To introduce the MQ nanogel into the PMPS liquid, a sample of the nanogel was dissolved in MTBE (10 mL) and mixed with the liquid using a PSB Gals ultrasonic bath for 10 min. Then the solvent was removed under vacuum at 1 mm Hg on a rotary evaporator until the constant mass at 90 °C.
Conclusions
While studying the polycondensation of tetraethoxysilane and methyldiphenylethoxysilane in acetic acid, the new phenyl-containing MQ nanogel was obtained and characterized. Its introduction into the PMPS liquid was shown to lead to an increase in the viscosity while maintaining the thermal and sealing characteristics. The observed thickening effect appeared to be quite high and opens up new prospects for the application of nanogels as molecular thickeners in siloxane compositions.
References
- S. A. Bhat, M. S. Charoo, Mater. Today: Proc., 2019, 18, 4416–4420. DOI: 10.1016/j.matpr.2019.07.410
- K. G. Binu, B. S. Shenoy, D. S. Rao, R. Pai, Proced. Mater. Sci., 2014, 6, 1051–1067. DOI: 10.1016/j.mspro.2014.07.176
- M. Hemmat Esfe, R. Esmaily, M. Khaje Khabaz, A. Alizadeh, M. Pirmoradian, A. Rahmanian, D. Toghraie, Tribol. Int., 2023, 178, 108086. DOI: 10.1016/j.triboint.2022.108086
- Y. Z. N. Нtwe, A. S. Al-Janabi, Y. Wadzer, H. Mamat, Friction, 2024, 12, 569–590. DOI: 10.1007/s40544-023-0774-2
- A. S. Lyadov, Y. M. Yarmush, O. P. Parenago, Russ. J. Appl. Chem., 2019, 92, 1805–1809. DOI: 10.1134/S107042721912023X
- C. A. Snyder, D. Mayotte, C. J. Donahue, J. Chem. Edu., 2006, 83, 902. DOI: 10.1021/ed083p902
- G. Mariani, in: Lubricant Additives. Chemistry and Applications, 3rd ed., L. R. Rudnick (Ed.), CRC Press, Boca Raton, 2017, ch. 6, pp. 473–484. DOI: 10.1201/9781315120621
- V. V. Sinitsyn, Grease Lubricants in the USSR: A Handbook, 2nd ed., Moscow, Khimiya, 1984 (in Russian).
- A. S. Lyadov, A. A. Kochubeev, O. P. Parenago, Petroleum Chem., 2023, 63, 618–623. DOI: 10.1134/S0965544123030027
- M. V. Sobolevsky, O. A. Muzovskaya, G. S. Popeleva, Properties and Areas of Application of Organosilicon Products, Moscow, Khimiya, 1975 (in Russian).
- M. V. Sobolevsky, I. I. Skorokhodov, K. P. Grinevich, Oligoorganosiloxanes. Properties, Production, Application, Moscow, Khimiya, 1985 (in Russian).
- G. G. Delides, Radiation Phys. Chem., 1980, 16, 345–352. DOI: 10.1016/0146-5724(80)90228-9
- G. C. Corfield, D. T. Astill, D. W. Clegg, ACS Symp. Ser., 1984, 266, 473–480. DOI: 10.1021/bk-1984-0266.ch024
- V. V. Lyashevich, N. I. Trofimova, O. F. Aleksashina, V. G. Shigorin, T. I. Sunekants, N. V. Oleinik, V. V. Severnyi, Polym. Sci. USSR, 1988, 30, 2021–2030. DOI: 10.1016/0032-3950(88)90055-X
- B. V. Molchanov, E. A. Chuprova, S. V. Vinogradov, A. A. Donskoi, N. V. Baritko, Klei, Germetiki, Tekhnol., 2005, 11, 16–20.
- I. B. Meshkov, A. A. Kalinina, V. V. Gorodov, A. V. Bakirov, S. V. Krasheninnikov, S. N. Chvalun, A. M. Muzafarov, Polymers, 2021, 13, 2848. DOI: 10.3390/polym13172848
- A. V. Bakirov, S. V. Krasheninnikov, M. A. Shcherbina, I. B. Meshkov, A. A. Kalinina, V. V. Gorodov, E. A. Tatarinova, A. M. Muzafarov, S. N. Chvalun, Polymers, 2022, 15, 48. DOI: 10.3390/polym15010048
- A. Y. Malkin, M. Y. Polyakova, A. V. Andrianov, I. V. Meshkov, A. M. Muzafarov, Phys. Fluids, 2019, 31, 083104. DOI: 10.1063/1.5116344
- A. Y. Malkin, M. Y. Polyakova, A. V. Subbotin, I. B. Meshkov, A. V. Bystrova, V. G. Kulichikhin, A. M. Muzafarov, J. Mol. Liquids, 2019, 286, 110852. DOI: 10.1016/j.molliq.2019.04.129
- M. V. Mironova, I. B. Meshkov, A. A. Shabeko, V. V. Shutov, V. G. Kulichikhin, E. A. Tatarinova, INEOS OPEN, 2020, 3, 29–34. DOI: 10.32931/io2004a
- I. B. Meshkov, A. A. Kalinina, V. V. Kazakova, A. I. Demchenko, INEOS OPEN, 2020, 3, 118–132. DOI: 10.32931/io2022r
- T. D.-F. López, A. F. González1, Á. D. Reguero, M. Matos, M. E. Díaz-García, R. Badía-Laíño, Sci. Technol. Adv. Mater., 2015, 16, 055005. DOI: 10.1088/1468-6996/16/5/055005
- S. V. Vinogradov, E. A. Polivanov, E. A. Chuprova, Plastmassy, 2019, 3–4, 59–65. DOI: 10.35164/0554-2901-2019-3-4-59-65
- S. V. Vinogradov, A. N. Polivanov, E. A. Chuprova, Khim. Prom. Segodnya, 2016, 1, 13–18.
- X. Li, Z. Cao, Z. Zhang, H. Dang, Appl. Surface Sci., 2006, 252, 22, 7856–7861. DOI: 10.1016/j.apsusc.2005.09.068
- W. Dai, B. Kheireddin, H. Gao, H. Liang, Tribol. Int., 2016, 102, 88–98. DOI: 10.1016/j.triboint.2016.05.020
- E. Y. Oganesova, A. S. Lyadov, O. P. Parenago, Russ. J. Appl. Chem., 2018, 91, 1559–1573. DOI: 10.1134/s1070427218100014
- I. E. Uflyand, V. A. Zhinzhilo, V. E. Burlakova, Friction, 2019, 7, 93–116. DOI: 10.1007/s40544-019-0261-y
- W. Yu, H. Xie, J. Nanomater., 2012, 435873. DOI: 10.1155/2012/435873
- J. Zhao, Y. Huang, Y. He, Y. Shi, Friction, 2021, 9, 891–917. DOI: 10.1007/s40544-020-0450-8
- M. Gulzar, H. H. Masjuki, M. A. Kalam, M. Varman J. Nanopart. Res., 2016, 18, 223. DOI: 10.1007/s11051-016-3537-4
- В. Badmaev, D. Makarova, A. Mashanov, U. Mishigdorzhiyn, Lubricants, 2023, 11, 9. DOI: 10.3390/lubricants11010009
- F. Ilie, C. Covaliu, Lubricants, 2016, 4, 12. DOI: 10.3390/lubricants4020012
- CN Patent, 104140535A, 2014.
- K. M. Borisov, A. A. Kalinina, E. S. Bokova, M. N. Ilyina, G. V. Cherkaev, E. A. Tatarinova, S. A. Milenin, A. V. Bystrova, M. Moeller, A. M. Muzafarov, Mendeleev Commun., 2022, 32, 164–166. DOI: 10.1016/j.mencom.2022.03.003
- E. V. Egorova, N. G. Vasilenko, N. V. Demchenko, E. A. Tatarinova, A. M. Muzafarov, Doklady Chem., 2009, 424, 15–18. DOI: 10.1134/S0012500809010042





