2023 Volume 6 Issue 2
|
INEOS OPEN, 2023, 6 (2), 40–43 Journal of Nesmeyanov Institute of Organoelement Compounds |
|
Effect of Carbon Dioxide on Bromantane Syntesis by Reductive Amination
without an External Hydrogen Source
a Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, str. 1, Moscow, 119334 Russia
b National Research University Higher School of Economics, ul. Miasnitskaya 20, Moscow, 101000 Russia
Corresponding author: D. Chusov, e-mail: chusov@ineos.ac.ru
Received 17 November 2023; accepted 6 December 2023
Abstract
The effect of carbon dioxide on the reaction of bromantane synthesis by reductive amination using carbon monoxide as a reducing agent has been studied. A nonlinear dependence of the yield of the target product on the ratio of gases was observed. It was found that, depending on the conditions, carbon dioxide could both decrease and increase the yield of bromantane.
Key words: carbon monoxide, carbon dioxide, reductive amination, bromantane.
Introduction
Nowadays amines play a crucial role in human life. One of the most important application fields of amines is pharmaceutical industry [1]. It was shown that nearly a quarter of C–N bond forming reactions in the pharmaceutical industry are performed via reductive amination [2]. Hence the development of new methods of reductive amination catches attention of scientists all over the world.
Among reductive processes, those utilizing molecular hydrogen play a significant role [3, 4]. The field of reductive amination is no exception [5, 6]. Some of the other most common reductive amination systems utilize sodium borohydride and its derivatives as reducing agents [5, 7]. In addition, there are systems that use other reducing agents, including, but not limited to, sodium hypophosphite [8], carbon monoxide [9, 10] and its mixtures with water [11–13] and other gases [14, 15], as well as different CO surrogates, such as iron pentacarbonyl [16] or formates [17–20].
Bromantane (ladasten) is a medicinal substance used as an antianxiety agent [21]. There are two main approaches to the synthesis of bromantane: reductive amination [22, 23] and palladium-catalyzed cross-coupling [24]. While palladium-catalyzed cross-coupling requires more expensive and difficult to obtain starting materials and shows mediocre results, reductive amination can be considered as a more affordable approach.
Recently our group has developed a method for the synthesis of pharmaceuticals via reductive amination using converter gas as a reductant [14]. We hypothesized that carbon dioxide, which is one of the components of converter gas, might be tuning the catalytic system.
Nowadays carbon dioxide has many applications. It is often used as a C1 building block [25], a CO source [26, 27], and a protective group [28, 29]. Other uses of carbon dioxide are related to its unique physical properties, one such example being the so-called supercritical drying [30]. It is also known that CO2 at elevated pressure can improve the solubility of various gases in materials [31, 32], which can be utilized for enrichment of reaction media with favorable gaseous substances, including carbon monoxide [33].
Therefore, we decided to study in detail the possibility of CO2 influence on the catalytic synthesis of bromantane via the reductive amination pathway using CO as a reducing agent.
Results and discussion
Firstly, we studied the effect of adding CO2 on the synthesis of bromantane and using CO as a reducing agent. The preliminary results showed a decrease in the catalytic activity upon addition of CO2 (Table 1, compare entries 1 and 2–5). However, when the CO2 pressure reached 40 bar, the catalytic activity of the system increased dramatically (entry 6). When the CO2 pressure was further increased, the yield of the target product began to decrease (entry 6 vs 7, 8). For clarity, these results are presented in Fig. 1.
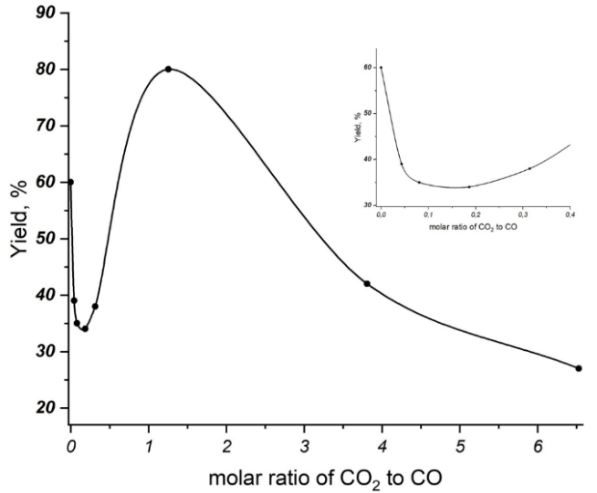
Figure 1. Dependence of the bromantane yield on the CO2/CO ratio.
Table 1. Investigation of the effect of adding CO2
|
Entrya
|
CO2 pressure, bar
|
CO2/CO molar ratio
|
Yield, %b
|
|
1
|
0
|
0
|
60
|
|
2
|
2.6
|
0.04
|
39
|
|
3
|
5
|
0.08
|
35
|
|
4
|
10
|
0.19
|
34
|
|
5
|
17.6
|
0.31
|
38
|
|
6
|
40
|
1.25
|
80
|
|
7
|
62
|
3.81
|
42
|
|
8
|
100
|
6.53
|
27
|
|
a Reaction conditions: 2 mol % of RuCl3, 1 eq. of 4-bromoaniline, 1.1 eq. of 2-adamantanone, CO pressure of 30 bar, corresponding pressure of CO2 (0–100 bar), 140 °C, 22 h; |
Three distinct trends in the phase behavior were identified that depend on the partial pressure of CO2 (Fig. 1). By comparing these trends, we have developed a hypothesis that explains the non-linear yield of the product based on the CO2/CO molar ratio.
During the reaction, CO is oxidized to CO2. Therefore, the addition of CO2 to the system can decrease the reaction yield by shifting the equilibrium toward CO, thus lowering the reductive potential of the system. This trend continues until the molar ratio of CO2 to CO reaches 0.1866.
However, as the CO2 partial pressure continues to increase (Fig. 2a, ρCO2 = 0.16 g/mL), a two-phase system is observed, consisting of a liquid phase, containing CO2, dissolved CO and acetonitrile, and a vapor phase, containing CO2, CO and MeCN saturated vapors. As the partial pressure increases, the fraction of the liquid phase rises due to the increasing MeCN/CO2 miscibility [34]. Reaching the reaction temperature (140 °C), our catalytic system remains biphasic, resulting in the highest yield of bromantane and activity. We assumed that such an increase might be due to the increased miscibility of MeCN with CO2. This process might be promoting higher CO ratio in the reaction mixture. It leads to the highest reaction rate due to the enhanced transport properties of the MeCN/CO2 system.
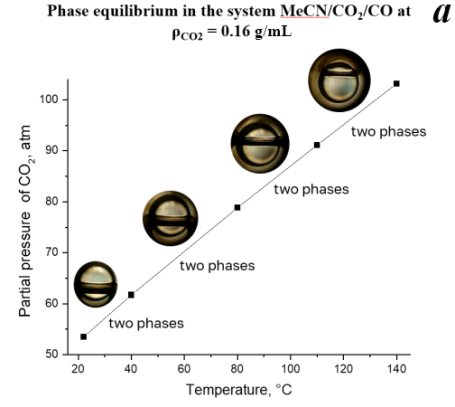
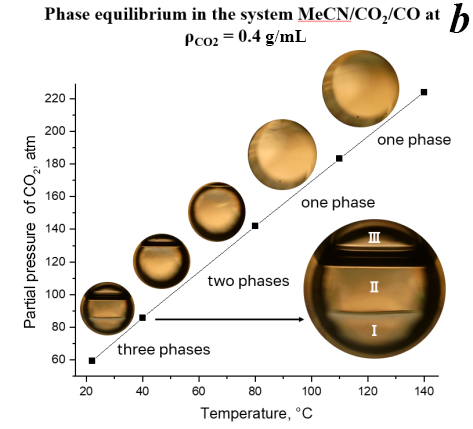
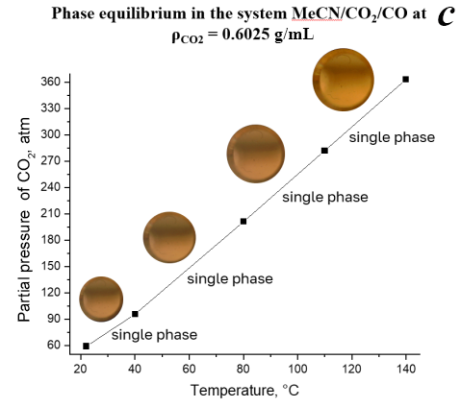
Figure 2. Phase state of the system MeCN/CO2/CO depending on the temperature and density of CO2 (0.16 g/mL (a), 0.4 g/mL (b), 0.6025 g/mL (c)).
A further increase in the CO2 ratio (Fig. 2b, ρCO2 = 0.4 g/mL) leads to a triple-phase system at room temperature, consisting of (I) MeCN-rich phase with dissolved CO2 and CO, (II) CO2-rich phase with dissolved MeCN and CO, and (III) MeCN/CO/CO2 saturated vapors. Upon heating to 40 °C, II–III phase boundary dissolves and the system transforms into a biphasic state containing highly swelled MeCN/CO2/CO phase and saturated vapors state. A further increase in the temperature (>80 °C) leads to the loss of the phase boundary. It is assumed that in this homogeneous system the effective interaction between the reagents and the catalyst is hindered due to the excessive CO2 content.
A further increase in the value of ρCO2 to 0.6025 g/mL (Fig. 2с) results in the excessive dilution of the medium even at room temperature and the system stays single-phase at temperatures up to 140 °C with a further decrease of the catalytic activity.
Furthermore, we studied the solubility of the compounds under consideration in CO2. We introduced a small amount of the substrate into an optical cell and filled it with CO2 (40 bar).
The results obtained suggest that only 4-bromoaniline can be dissolved in CO2 without acetonitrile or CO (Fig. 3 vs Figs. 4 and 5).
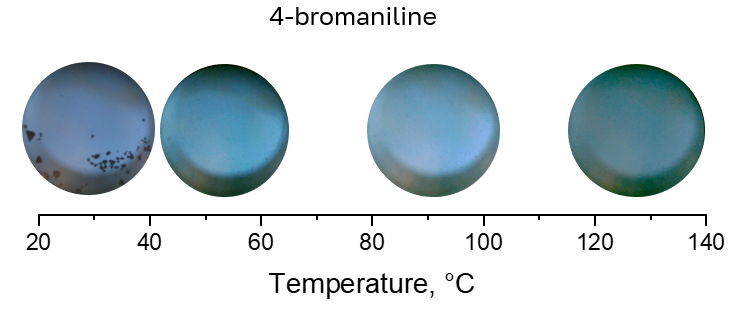
Figure 3. Solubility of 4-bromoaniline in CO2 (40 bar) at different temperatures.
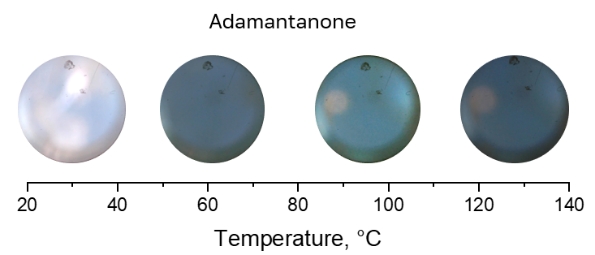
Figure 4. Solubility of 2-adamantanone in CO2 (40 bar) at different temperatures.
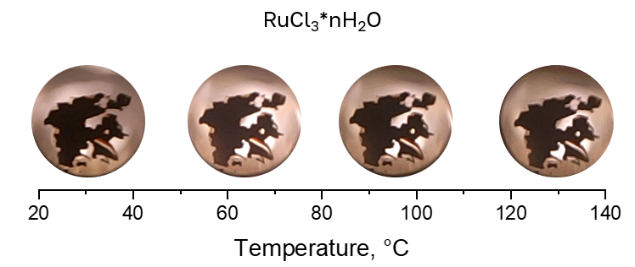
Figure 5. Solubility of RuCl3 in CO2 (40 bar) at different temperatures.
Experimental section
General remarks
Unless otherwise stated, all the reagents were purchased from commercial sources and used without further purification.
The 1H NMR spectra were recorded on Bruker Avance 400 or Varian Inova 400 spectrometers in CDCl3. The chemical shifts were measured relative to the solvent residual proton signals.
Low pressure (up to 30 bar) of carbon dioxide was achieved using a DINARC Plus cylinder pressure regulator (Gas Control Equipment, Czech Republic), a system of valves and high-pressure tubing (High Pressure Equipment Co, USA). High pressure (30–100 bar) of carbon dioxide was achieved using a P-50A high-pressure pump (Thar Technologies Inc., USA), a system of valves and high-pressure tubing (High Pressure Equipment Co, USA).
Syntheses
A glass vial placed in a 10 mL stainless steel autoclave was charged with 4-bromoaniline (52 mg, 0.303 mmol, 100 mol %), adamantanone (50 mg, 0.333 mmol, 110 mol %), ruthenium (III) chloride hydrate (1.58 mg, 6.05 µmol, 2 mol %), acetonitrile (0.32 mL), and 3 Ǻ molecular sieves (146 mg). The autoclave was sealed, connected to a carbon monoxide supply system, containing valves, manometers, and high-pressure tubing (Hy-Lok, South Korea), flushed three times with 10 atm of CO, and then charged with 30 bar of CO (99%, Lanthan CENTER FOR TECHNOLOGY). The autoclave was then connected to a carbon dioxide supply system [35, 36], consisting of a high-pressure pump (Thar, USA), valves and high-pressure tubing (High Pressure Equipment Co, USA), and filled with CO2 (99.997%, MGPZ) at the specified partial pressure of CO2 (30–100 bar, r.t.). In the case of the CO2 partial pressure of 1–30 atm, the autoclave was initially filled with CO2 via a system of a cylinder pressure regulator (Gas Control Equipment, Czech Republic) and a high-pressure tubing system (High Pressure Equipment Co, USA) followed by charging the reactor with CO using the above-mentioned procedure. After the reactor filling, it was placed into an oil bath with magnetic stirring (IKA, Germany) preheated to 140 °C for 22 h. After the reaction completion, the autoclave was cooled down and slowly depressurized. The reaction mixture was transferred into a vial and the autoclave was washed with dichloromethane (2×1 mL). In order to remove the catalyst, the reaction mixture was passed through a small pad of silica gel. The solvent was removed under reduced pressure and the residue obtained was analyzed by NMR spectroscopy.
Th phase equilibrium was studied using an in situ optical technique [36] based on the custom high-pressure set-up including a 3.3 mL stainless-steel optical cell with sapphire windows (Sitec, Switzerland) equipped with a temperature PID-controller (Termodat, Russia) and custom heating elements. For our studies, the cell was charged with 0.25 µL of acetonitrile (MeCN), sealed, filled with CO at 30 bar (corresponding density ρCO2 = 0.0225 g/mL) using the CO high pressure set-up. Then the system was filled with CO2 at different partial pressures (ρCO2 = 0.16, 0.4, or 0.6025 g/mL) via the high-pressure supply system [37]. After filling the cell with each substance, a total mass of the optical cell was measured on a GH-252 analytical balance (AND, Japan) with an accuracy of 0.001 g to specify the resulting CO/CO2/acetonitrile ratio. The cell was gradually heated to 140 °C; the images were taken each 5 min using a DigiMicro Prof USB-microscope (DigiMicro, Russia) and the CO/CO2/acetonitrile mix phase behavior was recorded using Pluggable Digital Viewer software (DigiMicro, Russia). The pressure–temperature correlations were calculated via the NIST Chemistry WebBook [38].
Conclusions
Hence, the effect of the addition of CO2 on the yield of bromantane in the catalytic reductive amination process involving CO was studied. The nonlinear dependence of the reaction yield on the CO2/CO molar ratio was revealed. Three main cases can be distinguished. First, the introduction of CO2 into the system initially reduces the yield by decreasing the molar fraction of CO. Second, a further increase in the CO2 partial pressure as well as its miscibility with acetonitrile promote higher miscibility of CO and acetonitrile, resulting in the highest yield. Third, a further dilution of CO2 has a negative effect as it excessively dilutes the medium and prevents efficient interaction of the reactants with the catalyst.
Acknowledgements
The NMR spectroscopic studies were performed with financial support from the Ministry of Science and Higher Education of the Russian Federation (agreement no. 075-00277-24-00) using the equipment of the Center for Molecular Composition Studies of INEOS RAS.
References
- O. I. Afanasyev, E. Kuchuk, D. L. Usanov, D. Chusov, Chem. Rev., 2019, 119, 11857–11911. DOI: 10.1021/acs.chemrev.9b00383
- S. D. Roughley, A. M. Jordan, J. Med. Chem., 2011, 54, 3451–3479. DOI: 10.1021/jm200187y
- S. A. Kuznetsova, S. M. Yunusov, A. S. Gak, V. I. Riazanov, Y. V. Nelyubina, R. Barker, M. North, V. P. Zhereb, E. A. Khakina, A. Naumkin, N. N. Lobanov, V. N. Khrustalev, D. Chusov, E. S. Kalyuzhnaya, Y. N. Belokon, ChemistrySelect, 2022, 7, e202203011. DOI: 10.1002/slct.202203011
- S. E. Lyubimov, V. A. Davankov, E. E. Said-Galiev, A. R. Khokhlov, Catal. Commun., 2008, 9, 1851–1852. DOI: 10.1016/j.catcom.2008.03.001
- T. Irrgang, R. Kempe, Chem. Rev., 2020, 120, 9583–9674. DOI: 10.1021/acs.chemrev.0c00248
- J. R. Cabrero-Antonino, R. Adam, M. Beller, Angew. Chem., Int. Ed., 2019, 58, 12820–12838. DOI: 10.1002/anie.201810121
- E. Podyacheva, O. I. Afanasyev, A. A. Tsygankov, M. Makarova, D. Chusov, Synthesis, 2019, 51, 2667–2677. DOI: 10.1055/s-0037-1611788
- F. Kliuev, A. Kuznetsov, O. I. Afanasyev, S. A. Runikhina, E. Kuchuk, E. Podyacheva, A. A. Tsygankov, D. Chusov, Org. Lett., 2022, 24, 7717–7721. DOI: 10.1021/acs.orglett.2c02807
- D. Chusov, B. List, Angew. Chem., Int. Ed., 2014, 53, 5199–5201. DOI: 10.1002/anie.201400059
- P. N. Kolesnikov, N. Y. Yagafarov, D. L. Usanov, V. I. Maleev, D. Chusov, Org. Lett., 2015, 17, 173–175. DOI: 10.1021/ol503595m
- A. Ambrosi, S. E. Denmark, Angew. Chem., Int. Ed., 2016, 12164–12189. DOI: 10.1002/anie.201601803
- S. A. Runikhina, M. A. Arsenov, V. B. Kharitonov, E. R. Sovdagarova, O. Chusova, Y. V. Nelyubina, G. L. Denisov, D. L. Usanov, D. Chusov, D. A. Loginov, J. Organomet. Chem., 2018, 867, 106–112. DOI: 10.1016/j.jorganchem.2017.11.003
- M. Viganò, F. Ragaini, M. G. Buonomenna, R. Lariccia, A. Caselli, E. Gallo, S. Cenini, J. C. Jansen, E. Drioli, ChemCatChem, 2010, 2, 1150–1164. DOI: 10.1002/cctc.201000044
- S. A. Runikhina, O. I. Afanasyev, E. A. Kuchuk, D. S. Perekalin, R. V. Jagadeesh, M. Beller, D. Chusov, Chem. Sci., 2023, 14, 4346–4350. DOI: 10.1039/D3SC00257H
- E. Podyacheva, A. I. Balalaeva, O. I. Afanasyev, S. A. Runikhina, O. Chusova, A. S. Kozlov, S. Liao, D. Chusov, New J. Chem., 2023, 47, 10514–10518. DOI: 10.1039/D3NJ01258A
- O. I. Afanasyev, D. L. Usanov, D. Chusov, Org. Biomol. Chem., 2017, 15, 10164–10166. DOI: 10.1039/C7OB02795H
- F. Ferretti, M. A. Fouad, F. Ragaini, Catalysts, 2022, 12, 106. DOI: 10.3390/catal12010106
- F. Ragaini, F. Ferretti, M. A. Fouad, Catalysts, 2023, 13, 224. DOI: 10.3390/catal13020224
- M. A. Fouad, F. Ferretti, F. Ragaini, J. Org. Chem., 2023, 88, 5108–5117. DOI: 10.1021/acs.joc.2c02613
- D. Formenti, F. Ferretti, F. Ragaini, ChemCatChem, 2018, 10, 148–152. DOI: 10.1002/cctc.201701214
- M. Mikhaylova, J. V. Vakhitova, R. S. Yamidanov, M. Kh. Salimgareeva, S. B. Seredenin, T. Behnisch, Neuropharmacology, 2007, 53, 601–608. DOI: 10.1016/j.neuropharm.2007.07.001
- I. S. Morozov, N. V. Klimova, L. N. Lavrova, N. I. Avdyunina, B. M. Pyatin, V. S. Troitskaya, N. P. Bykov, Pharm. Chem. J., 1998, 32, 1–4. DOI: 10.1007/BF02464216
- A. S. Babushkin, M. B. Navrotskii, I. A. Novakov, B. S. Orlinson, M. D. Robinovich, D. S. Sheikin, S. N. Voloboev, Pharm. Chem. J., 2017, 50, 781–787. DOI: 10.1007/s11094-017-1531-5
- A. D. Averin, M. A. Ulanovskaya, V. V. Kovalev, A. K. Buryak, B. S. Orlinson, I. A. Novakov, I. P. Beletskaya, Russ. J. Org. Chem., 2010, 46, 64–72. DOI: 10.1134/S1070428010010069
- C. S. Day, S. J. Ton, C. Kaussler, D. V. Hoffmann, T. Skrydstrup, Angew. Chem., Int. Ed., 2023, 135, e202308238. DOI: 10.1002/ange.202308238
- J. B. Jakobsen, M. H. Rønne, K. Daasbjerg, T. Skrydstrup, Angew. Chem., Int. Ed., 2021, 60, 9174–9179. DOI: 10.1002/anie.202014255
- C. Lescot, D. U. Nielsen, I. S. Makarov, A. T. Lindhardt, K. Daasbjerg, T. Skrydstrup, J. Am. Chem. Soc., 2014, 136, 6142–6147. DOI: 10.1021/ja502911e
- D. A. Guk, R. O. Burlutskiy, D. A. Lemenovskiy, E. K. Beloglazkina, Mendeleev Commun., 2023, 33, 14–16. DOI: 10.1016/j.mencom.2023.01.004
- A. G. Balybin, Y. M. Panov, L. V. Erkhova, D. A. Lemenovskii, D. P. Krut'ko, Mendeleev Commun., 2019, 29, 438–440. DOI: 10.1016/j.mencom.2019.07.028
- S. V. Balakhonov, S. Z. Vatsadze, B. R. Churagulov, Russ. J. Inorg. Chem., 2015, 60, 9–15. DOI: 10.1134/S0036023615010027
- A. Wong, L. H. Mark, M. M. Hasan, C. B. Park, J. Supercrit. Fluids, 2014, 90, 35–43. DOI: 10.1016/j.supflu.2014.03.001
- K. S. Stamer, M. A. Pigaleva, A. A. Pestrikova, A. Y. Nikolaev, A. V. Naumkin, S. S. Abramchuk, V. S. Sadykova, A. E. Kuvarina, V. N. Talanova, M. O. Gallyamov, Molecules, 2022, 27, 7261. DOI: 10.3390/molecules27217261
- Z. K. Lopez-Castillo, S. N. V. K. Aki, M. A. Stadtherr, J. F. Brennecke, Ind. Eng. Chem. Res., 2006, 45, 5351–5360. DOI: 10.1021/ie0601091
- T. S. Reighard, S. T. Lee, S. V. Olesik, Fluid Phase Equilib., 1996, 123, 215–230. DOI: 10.1016/S0378-3812(96)90029-1
- A. Yu. Alentiev, S. V. Chirkov, R. Yu. Nikiforov, N. A. Belov, A. M. Orlova, A. A. Kuznetsov, A. S. Kechekyan, P. A. Kechekyan, A. Yu. Nikolaev, Membr. Membr. Technol., 2022, 4, 162–169. DOI: 10.1134/S2517751622030027
- M. S. Rubina, A. A. Pestrikova, P. S. Kazaryan, A. Y. Nikolaev, I. S. Chaschin, N. A. Arkharova, A. V. Shulenina, M. A. Pigaleva, J. CO2 Util., 2022, 62, 102106. DOI: 10.1016/j.jcou.2022.102106
- E. E. Said-Galiev, L. N. Nikitin, A. Yu. Nikolaev, Yu. E. Vopilov, I. A. Garbuzova, A. R. Khokhlov, V. M. Buznik, Polym. Sci., Ser. A, 2015, 57, 271–278. DOI: 10.1134/S0965545X15030141
- H. Y. Afeefy, J. F. Liebman, S. E. Stein, Neutral Thermochemical Data in NIST Chemistry WebBook, NIST Standard Reference Database Number 69. DOI: 10.18434/T4D303





