2022 Volume 5 Issue 6
|
INEOS OPEN, 2022, 5 (6), 150–157 Journal of Nesmeyanov Institute of Organoelement Compounds |
|
On the Conversion of CO2 to Methanol: Challenges and Possible Solutions. Highlights
a Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, str. 1, Moscow, 119334 Russia
b Tver State Technical University, nab. Afanasiya Nikitina 22, Tver, 170026 Russia
Corresponding author: Z. B. Shifrina, e-mail: shifrina@ineos.ac.ru
Received 15 June 2023; accepted 16 August 2023
Abstract
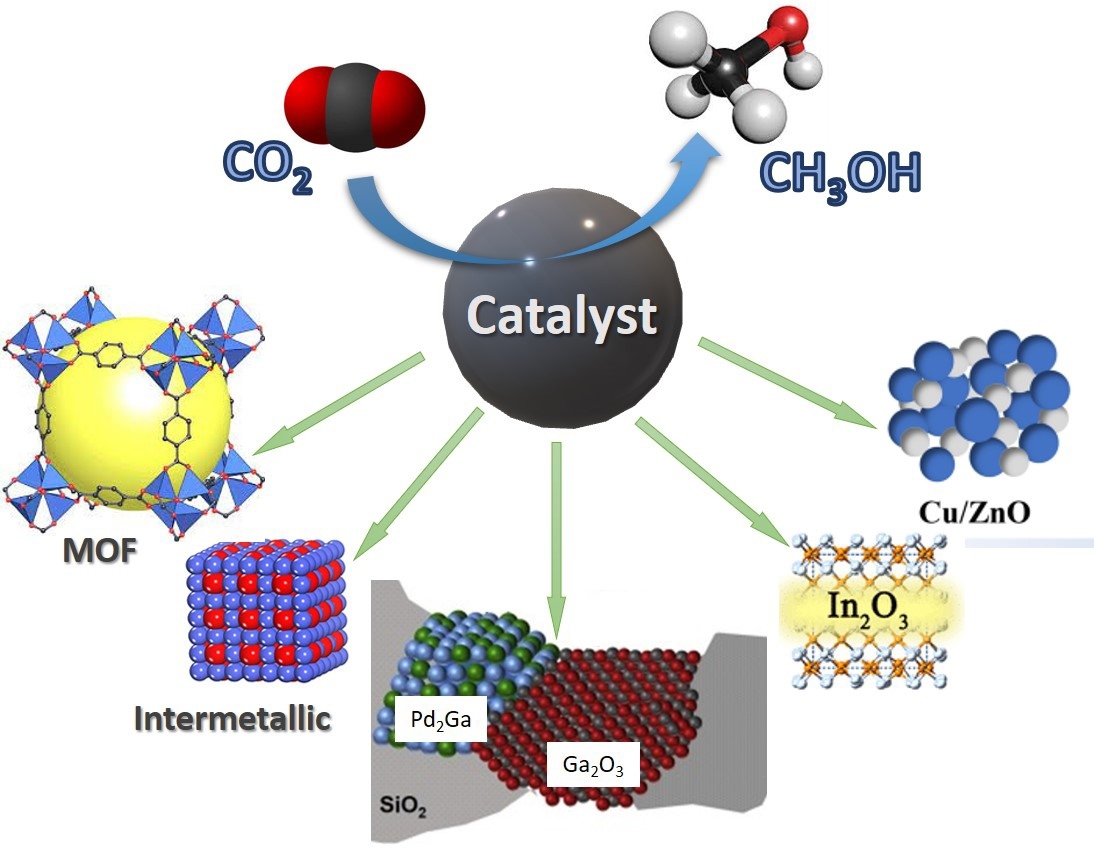
The state-of-the-art and prospects of carbon dioxide hydrogenation to methanol via heterogeneous catalysts are highlighted. The main focus is on various catalysts including metals, metal oxides, and intermetallic compounds. The current efforts are directed to the development of catalytic structures that allow for the tuning of the catalyst composition and modulation of surface structures and potentially provide promising catalytic performance.
Key words: methanol synthesis, CO2 hydrogenation, heterogeneous catalysts, metal–organic frameworks.
Introduction
Carbon dioxide, being the main component of greenhouse gases, is a source of both positive and negative effects on human life. On the one hand, the presence of CO2 along with other greenhouse gases creates conditions for a comfortable temperature environment for life. On the other hand, excessive combustion of fossil fuels causes a continuous increase in the concentration of CO2 in the atmosphere, which leads to significant and probably irreversible changes in the global climate.
A reduction of CO2 emissions is an urgent problem, a breakthrough in solving which will allow for responding to the challenges of ecological economics [1].
Significant progress in energy-efficient catalytic conversion of CO2 using renewable energy sources has the potential to reduce CO2 emissions and the dependence on fossil resources such as oil [2]. The CO2 molecule is thermodynamically and chemically stable. The application of CO2 as the only reagent requires a lot of energy [3]. Nevertheless, the process becomes thermodynamically more favorable if another compound with a higher Gibbs free energy is introduced as a co-reactant, for example, H2 [3].
Hence, CO2 hydrogenation is one of the promising approaches to the use of CO2 as a carbon source to produce value-added products, such as alcohols, dimethyl ether, as well as various hydrocarbons (olefins, liquid hydrocarbons, and aromatic compounds) [4–13].
Methanol (MeOH) is an important chemical raw material and can be used as a fuel for internal combustion engines and fuel cells. Therefore, converting CO2 to methanol is one of the most attractive and potentially beneficial processes and should play an important role in reducing CO2 emissions and creating a new carbon cycle.
Owing to the similarity of the processes of conversion of synthesis gas into methanol and the synthesis of methanol from CO2, copper-based catalysts gained widespread use. These catalysts along with the general aspects of CO2 conversion are thoroughly discussed in the literature [14–24]. Although the relationship between the structure of a catalyst and its activity in this process is known, debate continues on its mechanism and identification of the active sites.
It is well known that these catalysts have a short life cycle and low activity at low temperatures, when the synthesis of methanol is thermodynamically favorable [25–27]. To solve the problem of creating active catalytic systems, the catalysts based on noble metals were studied, which, however, showed low selectivity towards methanol. Bimetallic oxides such as ZnO/ZrO2 were shown to be active and economically promising catalysts for the efficient methanol synthesis [28]. More importantly, these catalysts exhibit good stability even in the presence of sulfur compounds such as H2S and SO2.
Recent years have witnessed growing attention to the development of catalysts based on main group metals. For example, the catalysts based on In2O3 exhibit high activity, selectivity, and stability [29, 30]. Intermetallic compounds offer an alternative to the bimetallic catalytic systems, especially in terms of controlling the formation of favorable active sites with similar metal ratios and structural stability [31].
In this brief review, we focus on the recent advances in the development of heterogeneous catalysts based on transition metals, their oxides, and main group metals for the conversion of CO2 to methanol and methods for their production. The catalysts derived from indium oxide (In2O3), intermetallic compounds (Ni-Ga), and new nanostructured catalysts based on metal-organic frameworks (MOFs), which act as an alternative to the conventional systems, are considered separately [32]. The possible prospects for the application of certain heterogeneous catalytic systems in the process of methanol synthesis from CO2 are evaluated.
Structural characteristics of the catalysts and their effect on the catalytic activity
The hydrogenation of carbon dioxide to methanol is extremely sensitive to the structural characteristics of a catalyst. In particular, the catalytic activity strongly depends on the parameters such as the distribution of metal particles on the surface and their surface area; size, composition, and electronic properties of the catalytic particles, their alloys, as well as the chemistry of their interface; adsorption of reactants on the catalytic composite and mass transfer efficiency. As a rule, the variation of these parameters is achieved by introducing various promoters into the catalyst, selecting a support, and choosing an optimal method for the catalyst production.
The effect of particle sizes was demonstrated using the most studied catalyst based on copper and zinc. It was found that the smallest ZnO particles have a promoting effect on the catalyst activity. Being the least thermodynamically stable, such ZnO particles are capable of migrating to the copper surface and being reduced to atomic Zn under the conditions of a catalytic reaction [33]. This ensures the most complete distribution of zinc over the surface of Cu nanoparticles, which leads to an increase in the activity. Therewith, the size of copper nanoparticles should be at least 10 nm, and its decrease, on the contrary, leads to a decrease in the activity [33, 34]. Furthermore, of particular significance is the surface area of Cu NPs, which should be in the range of 18–23 m2/g [23]. In addition to the sizes of the NPs, the hydrogenation of CO2 to methanol is also structurally sensitive. A comparison of the activity of Cu(100) [35], polycrystalline copper foil [36], and Cu(110) [37] revealed a higher activity of Cu(110) in the metallic form. A similar dependence was observed by Palomino et al. [38]: the activity decreased in the order Cu(111) < Cu(100) < ZnO/Cu(111) < ZnO/Cu(100).
Alkaline [39] and alkaline earth metals [40], rare earth [41], transition, and also main group elements [42, 43] are used as promoters to increase the activity of the main metal in the reaction. Their role consists in improving the dispersion of metal NPs, increasing the surface area, changing the adsorption properties of the surface, primarily affecting the processes of hydrogen transfer and adsorption, and forming additional oxygen vacancies.
An important aspect is the formation of an interface between two metals when using bimetallic catalysts. Thus, it is the Cu-ZnO interface that is believed to be the region where the catalytic reaction occurs in the case of the Cu-ZnO-Al2O3 bimetallic catalyst [44]. The DFT calculations showed that the presence of atomic Zn and its clusters (Zn3) at the Cu-ZnO interface significantly reduces the activation barrier for the hydrogenation process [45, 46]. Another study [47] demonstrated that Cu+-O-Zn compounds are formed at the Cu-Zn interface during the CO2 hydrogenation, which stabilize the reaction intermediates, namely, formates and methoxy-substituted compounds. Another factor contributing to an increase in the activity upon the formation of a bimetallic system is the appearance of oxygen vacancies, which are also concentrated at the interface between two metals. Oxygen vacancies activate the CO2 molecule and also stabilize intermediates [29, 48–50]. The importance of the presence of oxygen vacancies was demonstrated in a number of reports [51–53], but the most striking example is the catalyst based on In2O3, the activity of which was first predicted by the computational methods [54] and then confirmed experimentally [29, 30, 48, 49, 55]. It is the presence of a large number of oxygen vacancies on the surface of pure In2O3 that determines its activity in the reaction. The introduction of a second metal component, namely, Au, Pd, Ni, Ir [48, 51, 56–58], and others contributes to an increase in the number of oxygen vacancies and an increase in the activity of the catalytic system.
Most of the catalysts studied in the CO2 hydrogenation are three-component systems in which two metals are immobilized on a support. The most commonly used substrates are metal oxides, such as Al2O3 [22, 59], ZrO2 [28, 55, 60], CeO2 [61, 62], as well as SiO2 [43], and various organic and organometallic supports [63]. The role of the support is to increase the stability of the catalytic system, structural segregation of metal particles, additional electronic and structural interactions that can promote activity, and change the textural characteristics of the catalyst to facilitate diffusion and mass transfer processes. The effect of strong metal–support interaction is known that has a strong influence on the morphology, chemical state, stability and activity of catalytic particles [64]. For example, a comparison of the activity of the copper catalysts with various supports (CeO2, ZnO, and ZrO2) revealed the highest activity of the Cu-CeO2 catalytic system due to the presence of strong interactions between the metal and the support, which ensured the more efficient dispersion of CuO particles over the support surface [61]. Similarly, due to such interactions, the copper catalyst based on spinel ZnFe2O4 as a support showed high activity [65]. The spinel structure promoted the formation of small ZnO particles located at the interface with copper particles, which had a positive effect on the catalytic process.
Besides stabilization and improved dispersion of metal particles, the support can increase the catalytic activity of the composite owing to the presence of oxygen vacancies [66–68]. This was demonstrated for the Cu-CeO2 catalyst [61] and a number of platinum-based catalysts. Thus, the activity of the Pt/TiO2 catalyst exceeded that of Pt/SiO2 [69]. The authors associated the effect with the presence of oxygen vacancies in the TiO2 support.
The carrier can have an additional promoting effect due to the presence of acidic/basic sites. Acidic sites promote CO2 adsorption, while basic sites facilitate the hydrogenation of intermediates [70].
It is worth noting that the structure of the catalyst changes dynamically during the reaction. This was comprehensively studied using the Cu/ZnO/Al2O3 catalyst as an example. These changes include the formation of partially oxidized Znẟ+ compounds at defective sites in the copper lattice, partial migration of Zn into the Cu lattice, formation of a Cu-Zn alloy, as well as the formation of ZnO layers on the copper surface [44, 71–73]. Under severe reaction conditions, irreversible changes occur also in the catalyst structure, which have a negative effect on the catalytic activity. Thus, a decrease in the activity of Cu/ZnO/Al2O3 is associated with the aggregation of ZnO and sintering of copper particles [74], as well as the oxidation of Cu0 to Cu2+. A similar mechanism for a decrease in the activity, associated with the aggregation of particles during the reaction, was also described for other catalytic systems. In particular, the catalysts based on In2O3 are susceptible to aggregation [56, 75]. An important reason that causes significant aggregation of particles during the reaction, in addition to high temperatures and pressure, is the presence of water vapor in the system. Water results from the reverse water–gas shift reaction—one of the side reactions of CO2 hydrogenation, which produces CO and H2O. It is possible to minimize the structural changes in the catalyst and its deactivation by selecting a suitable support, introducing a structural promoter or a hydrophobic component [29, 64, 76]. Thus, the ZrO2 support has proven itself well for indium-based catalysts [29, 49, 55], the introduction of which enabled the reduction of In2O3 sintering and the formation of large agglomerates after the reaction. The introduction of noble metals (Au, Pd) allowed for increasing the thermal stability of particles by increasing the Hüttig temperature and, as a consequence, reducing particle sintering during the reaction [53, 56, 58].
Table 1 presents the comparative data on the activities of some catalysts which will be discussed in detail below.
Table 1. Catalytic activity of some catalysts in the hydrogenation of CO2 to methanol
|
Catalyst
|
P (MPa)
|
T (K)
|
CO2:H2
|
MeOH, mol·kg cat–1 h–1
|
Selectivity, %
|
Ref.
|
|
Cu/Al2O3
|
10
|
473
|
1:3.8
|
1.05
|
46.2
|
[40]
|
|
Cu-K/Al2O3
|
10
|
473
|
1:3.8
|
1.62
|
62.2
|
[40]
|
|
Cu-Zn-Zr-La
|
3
|
503
|
1:3
|
2.7
|
49.8
|
[41]
|
|
Cu/Ti@SiO2
|
2.5
|
503
|
1:3
|
2.92
|
85.0
|
[77]
|
|
Cu/SiO2
|
2.5
|
503
|
1:3
|
0.60
|
49.0
|
[77]
|
|
Cu-Zn
|
3
|
513
|
1:3
|
1.39
|
36.5
|
[78]
|
|
CuO-ZnO-ZrO2
|
3
|
513
|
1:3
|
1.91
|
41.6
|
[79]
|
|
Pd-Cu/CeO2
|
4.1
|
523
|
1:3
|
1.37
|
28.4
|
[80]
|
|
Pd-Cu/ZrO2
|
4.1
|
523
|
1:3
|
1.87
|
26.8
|
[80]
|
|
Pd-Cu/Al2O3
|
4.1
|
523
|
1:3
|
1.69
|
31.4
|
[80]
|
|
Pt-Co/Al2O3
|
3.2
|
425
|
1:3
|
74.9
|
–
|
[81]
|
|
Pt-Co/SiO2
|
3.2
|
425
|
1:3
|
30.2
|
–
|
[81]
|
|
In2O3/ZrO2
|
5.0
|
573
|
1:4
|
9.22
|
99.8
|
[29]
|
|
Pd-P/In2O3
|
5.0
|
573
|
1:4
|
27.81
|
70
|
[51]
|
|
In/SiO2
|
3.0
|
553
|
1:3
|
2.56
|
89.0
|
[82]
|
|
Cu-In/SiO2
|
3.0
|
553
|
1:3
|
13.7
|
78.1
|
[82]
|
|
Ni-Ga/SiO2
|
0.1
|
483
|
1:3
|
7.50
|
–
|
[83]
|
Methods for the production of catalysts play a significant role in the creation of active catalytic systems. For example, for the Cu-Zn catalysts, impregnation is the simplest approach, but does not allow for obtaining the catalysts with a high metal content, >10–20% [84]. At the same time, deposition can produce crystalline precursors, which are ultimately formed into uniformly distributed homogeneous metal nanoparticles on a support [85, 86]. Deposition can afford the systems with a greater metal loading, but, at the same time, can also cause the problem of controlling the growth of nanoparticles. However, due to its simplified procedure and economic viability, there is continuous interest in developing this method for industrial applications.
There are a number of other methods for the production of catalysts, such as the sol–gel method [87], the template method for the synthesis of colloid crystals [60], the liquid reduction method [88], the microwave method [89], and the ammonia evaporation method [90, 91]. Based on the well-established composition–structure–activity relationships of the catalyst, these methods can control the catalytic activity by varying the surface area of Cu NPs, metal dispersion, particle sizes, metal/metal oxide interaction at the interface, and metal–substrate interaction.
Copper-based catalysts
As it was mentioned earlier, copper catalysts are among the most explored catalysts for converting CO2 to methanol. At the same time, copper catalysts feature low stability and a short life cycle, as well as low activity at lower temperatures, when methanol synthesis, on the contrary, is thermodynamically more favorable [25–27]. To address this issue, various bimetallic catalysts as well as the catalysts immobilized on different supports have been developed. The variation of these parameters allowed for achieving a significant increase in the activity, selectivity, and stability of the catalytic systems.
Main group metal oxides are likely to be the most commonly used modifiers that allow for tuning the metal–metal oxide interface and associated electronic and structural effects, improving the ability of copper to reduce, and increasing the adsorption properties. In addition to the introduction of zinc oxide, many reports consider the effect of the simultaneous introduction of several oxides into the system. Thus, good results were obtained using the Cu-ZnO-ZrO2-SiO2 catalyst [43]. The introduction of ZrO2, Ga2O3, and Cr2O3 additives into the structure of the conventional Cu-ZnO-Al2O3 catalyst increased the activity by optimizing the Cu+/Cu0 ratio [92, 93].
The effect of an alkaline promoter on the Cu/Al2O3 catalyst was studied [40]. It was found that the modification with Ba and K significantly improves the adsorption of CO2 on the catalyst surface. However, the catalysts had different selectivities towards methanol. While K improved the synthesis of methanol, Ba promoted the water–gas shift reaction, resulting in the formation of a significant amount of CO during the reaction. Rare earth metals also displayed different effects on the productivity of the catalytic system [41]. The introduction of La and Ce into the Cu/Zn/Zr catalyst increased the methanol yield, while Nd and Pr, in contrast, decreased it. The authors attributed this behavior to the stronger interaction of La and Ce with the support, resulting in increased hydrogen transfer efficiency. The Cu-Ni/CeO2 bimetallic catalyst showed good results on selectivity [94]. The partial reduction of Ce during the reaction, Ce4+ → Ce3+, led to the appearance of oxygen vacancies, facilitating the adsorption and activation of CO2. Noble metals, Au and Ag, also have a positive effect on methanol yield [95, 96]. Gold increased the thermal stability of copper particles and prevented their sintering during the reaction, while the formation of the Au–Cu interface promoted the reduction of copper.
Catalysts based on noble metals (Pd, Pt)
Palladium and platinum catalysts immobilized on a solid support are active in the formation of MeOH during CO hydrogenation at low temperatures [97]. Pd catalysts supported on La2O3 [98], Nd2O5 [98], and CeO2 [99] can selectively convert CO into MeOH with high yield at 170 °C and 3.0 MPa, while the Cu-ZnO catalyst requires a higher temperature (for example, 230 °C) to obtain comparable amounts of MeOH [99]. Based on these results, it was suggested that Pd- and Pt-based catalysts could be effective candidates for the low temperature hydrogenation of CO2 to methanol, when methanol formation is thermodynamically more favorable. However, the computational and experimental studies showed that Pt nanoparticles cannot catalyze the reaction due to weak CO2 binding [100]. Hence, the CO2 adsorption and activation are major challenges in the development of noble metal catalysts for the hydrogenation of CO2 to methanol. The problem of weak CO2 adsorption can be overcome in several ways. One way is to change the basicity of the catalyst surface, for example, using Ca and Mg oxides, which have moderate basicity [101]. The additives with moderate basicity, such as CaO and MgO, promote methanol formation balancing between the adsorption and hydrogenation of formates on the surface.
Metal oxides as supports for Pd can also improve the adsorption and activation of CO2 [102]. The key is to control the interaction between Pd and the oxide at the interface so that the Pd(0) and Pdδ+ active sites coexist [92]. ZnO and Ga2O3 are good supports owing to the formation of the corresponding Pd-Zn and Pd-Ga alloys with efficient electron transfer [103, 104], as well as the stabilization of Pdδ+ on the surface by GaxOy [92]. Pd and Zn alloys were shown to increase the rate of methanol formation [105, 106] at low pressure, 2 MPa. To improve the CO2 adsorption capacity and metal partitioning, the effect of a support on the catalytic properties of Pd-Cu bimetallic catalysts, including commercially available TiO2, CeO2, ZrO2, Al2O3, and CeO2, was investigated [80]. The rate of formation of MeOH over TiO2 and ZrO2 was 60% higher than in the case of a similar catalyst immobilized on SiO2.
Pt and Co alloys also demonstrated activity in the synthesis of methanol from CO2 at different ratios of Pt and Co [81]. The catalysts enriched with Pt on the surface showed better results. The critical role of the support was established and the promoting effect of the carbon support was shown to be due to the improved chemisorption/activation of reagents owing to a large number of unpaired electrons on the carbon support.
Catalysts based on main group metals
Recently, the catalytic properties of In2O3 have been discovered [29]. Owing to its basicity, the catalyst provides high selectivity in the formation of MeOH. The activity of In2O3 can be improved by the application of a carrier, for example, ZrO2, the role of which consists in maintaining the number of oxygen vacancies due to electronic interactions at the interface with In2O3 and preventing the sintering of In2O3 particles.
The promising properties of In2O3 catalysts are facilitating the development of large-scale selective synthesis of MeOH from CO2. The extremely high selectivity for methanol is reviving interest in converting CO2 to various hydrocarbons using methanol as an intermediate. In2O3 can be used with zeolites to form bifunctional catalysts for the synthesis of hydrocarbons from CO2, such as light olefins (C2–C4) [107, 108], gasoline hydrocarbons (C5+) [109], and aromatic hydrocarbons [12].
To further enhance the activity of In2O3 catalysts, the main strategy is to introduce dopants to improve H2 adsorption and create interfacial sites for the CO2 adsorption and hydrogenation [110]. Due to their strong affinity to H2, transition metals, in particular noble metals, are potential candidates for the application as doping agents [56, 111–113]. In addition to Pd and Pt noble metals, Cu was used as a dopant in the In-Zr catalysts through the production of a series of mixed oxides during the synthesis of methanol [55]. The activity of the catalysts varied depending on the composition of the catalyst, and the composite Cu0.25-In0.75-Zr0.5-O showed the best results among other mixed oxide catalysts, while significantly exceeding in the activity the reference Cu-ZrO catalyst.
Besides the additives designed to improve hydrogen adsorption, CO2 affinity additives are also introduced to improve the CO2 adsorption and activation. Thus, yttrium and lanthanum included in the In2O3/ZrO2 catalysts contributed to improving the activity of the catalyst under relatively mild reaction conditions [114]. These additives increase the number of oxygen vacancies by 60–70%, which, in turn, leads to the improved CO2 adsorption.
Intermetallic catalysts
Another type of heterogeneous catalysts for the synthesis of methanol from CO2 are intermetallic compounds (IMCs). These compounds have the following advantages over the alloy-based catalysts: (i) the bimetallic atomic ratio in the IMC is constant; (ii) IMCs are structurally stable owing to the covalent nature of metal–metal interactions; (iii) the nature of adjacent atoms enables modification of the electronic structure of active metals [115]. Despite these advantages, stability and reproducibility are the main challenges for producing uniform IMC NPs with controlled size and morphology [116].
The intermetallic compounds based on Ga were reported that allowed for obtaining stable bimetallic catalysts with a controlled and uniform atomic ratio [117]. It was shown that Pd supported on Ga2O3 is significantly more active in the synthesis of MeOH compared to the catalysts supported on other oxide materials, such as Al2O3, Cr2O3, TiO2, and ZrO2 [92]. This effect is partially explained by the formation of Pd-Ga intermetallic compounds after reduction, which provides an excess of atomic hydrogen, in turn, facilitating the suppression of MeOH decomposition and CO formation [118].
Catalysts based on MOFs
The development of highly efficient catalysts for the hydrogenation of CO2 to methanol requires the elucidation of the catalyst structure at the nanoscale level simultaneously with fine control of the active sites. Metal-organic frameworks (MOFs) are a class of crystalline nanoporous materials that are characterized by the high tunability owing to large accessible surface areas, pore functionality, and accessible reactive catalytic sites [119].
Since the catalytic activity in the methanol synthesis is directly related to the size and composition of metal/metal oxide composites, MOFs have an advantage in this process as they have the potential to interact with active metals and limit particle/crystallite size through nanosized secondary building blocks, as well as the possibility of changing the composition. In addition, the MOF structure is able to limit the growth of encapsulated metal NPs, protecting catalysts from deactivation caused by aggregation/agglomeration [120].
There are two known synthetic approaches to composite catalysts based on MOFs: bottom-up and top-down. The first method is similar to impregnation, which consists in the introduction of metal precursors into the pores of a metal-organic framework followed by pyrolysis; the second method requires encapsulation of NPs into a scaffold during MOF synthesis. The latter method allows the formation of composite materials with controlled functionalities, such as crystalline facets and morphology, but the synthetic procedure is more complicated [121].
Often the pre-synthesized metal nanocrystals are sensitive to oxygen and air moisture and decompose during their impregnation into the framework; therefore, the preparation of these catalysts requires special precautions. In this respect, the bottom-up method seems to be more promising for the preparation of catalytic systems active in industrial syntheses, including CO2 hydrogenation. Furthermore, the results of catalytic tests did not show superiority in the activity of the samples obtained using the top-down scheme [122, 123]. In contrast, the samples obtained by the bottom-up method showed outstanding results in terms of the reaction selectivity towards methanol [124–128] and significant activity. In particular, the activity of the Pd@ZIF-8 catalyst was superior to that of other known palladium catalysts [124]. In addition, the ease of modification of metal-organic frameworks allows for directed changes in the structure and investigation of the influence of ligands, metal substitution, and various electronic effects on the activity of the catalytic system [128].
The results obtained from investigations on the catalysts based on MOFs showed that the key parameters that lead to an increase in the activity of catalysts in the synthesis of methanol are the interaction of the metal with MOFs and ligand substitution, which requires further systematic study.
Conclusions and future outlook
The development of competitive catalytic technologies for the selective catalytic hydrogenation of CO2 into methanol opens the way to a carbon neutral society that could convert CO2 emissions into fuels and chemicals. Since the early 1990s, the transformation of CO2 into value-added products has been the focus of multiple studies, and much efforts have been aimed at developing efficient catalysts.
Various heterogeneous catalysts ranging from transition metals/metal oxides to main group metals/metal oxides have been explored in the last decade for the thermocatalytic conversion of CO2 to MeOH. Particular attention is drawn to the catalysts based on copper, noble metals, and main group metal oxides. Thus, the Cu-ZnO catalyst has been tested in the production of methanol from synthesis gas or CO2 for several decades; however, we still do not know in detail the key factors that determine its activity and selectivity: the origin and nature of the active site, as well as the reaction mechanism under actual conditions. The copper-based catalysts have so far demonstrated the best rates of methanol formation. However, the high selectivity (>80 C-mol %) can be achieved only at a significant reduction in the rate of methanol formation. In turn, the noble metal catalysts show a reverse trend. A higher selectivity can be achieved even at low temperatures, but this is accompanied by the low activity (rate). Recently suggested catalysts In2O3 and ZnO generally provide higher methanol selectivity than the copper-based counterparts even at comparable production rates; however, the operating temperature is higher, which leads to the higher energy consumption. The MOF catalysts exhibit the methanol selectivity comparable to those of In2O3 and ZnO, although the reaction temperature is generally lower.
Currently, with the development of nanotechnologies, increasing attention is paid to the development of catalysts with a given structure at the nanoscale level or even at the atomic level, such as the systems based on MOFs. The extensive mechanistic studies are carried out to identify possible reaction pathways, discover key intermediates, rate-determining steps and structure–activity relationships, as well as to identify the key factors that influence the product selectivity in the synthesis of methanol from CO2 using optimal catalyst systems.
To properly understand the process and mechanisms operating during the selective hydrogenation of CO2 to methanol, in addition to the experimental studies, the theoretical and modeling investigations are also necessary. Determining the reaction mechanisms at the molecular level opens up new possibilities for developing various reaction pathways already on the catalytic surfaces, which, in turn, will facilitate the creation of systems with active/selective sites for the synthesis of methanol under real conditions.
To summarize the results presented, we can conclude that, while new catalysts are being actively developed for the synthesis of methanol from CO2, further optimization is required for the classical Cu-ZnO catalysts. It is important to address issues such as improving low-temperature activity, deactivation during operation, and by-product formation. In this respect, the accumulation of an extensive array of experimental and theoretical work is important in the search for new catalysts and their optimization for the synthesis of methanol from CO2. Databases including information on the syntheses, active phases, promoters, activities, and stability, among others, are useful to avoid unnecessary repetitive work and to critically evaluate the practical viability of alternative catalyst compositions. It is obvious that among the alternative catalysts, the systems based on Pd, Au, and In2O3 have sufficient potential to overcome the limitations observed for the conventional Cu-ZnO catalysts. However, new systems still require significant improvements to meet the requirements for industrial applications of catalysts for the hydrogenation of CO2 to methanol.
Acknowledgements
This work was supported by the Russian Science Foundation, project no. 22-43-02025.
References
- J. P. Holdren, Science, 2008, 319, 424–434. DOI: 10.1126/science.1153386
- B. M. Tackett, E. Gomez, J. G. Chen, Nat. Catal., 2019, 2, 466. DOI: 10.1038/s41929-019-0284-9
- C. Song, Catal. Today, 2006, 115, 2–32. DOI: 10.1016/j.cattod.2006.02.029
- L. C. Grabow, M. Mavrikakis, ACS Catal., 2011, 1, 365–384. DOI: 10.1021/cs200055d
- W. Wang, S. Wang, X. Ma, J. Gong, Chem. Soc. Rev., 2011, 40, 3703–3727. DOI: 10.1039/C1CS15008A
- M. B. Ansari, S.-E. Park, Energy Environ. Sci., 2012, 5, 9419–9437. DOI: 10.1039/C2EE22409G
- M. M.-J. Li, S. C. E. Tsang, Catal. Sci. Technol., 2018, 8, 3450–3464. DOI: 10.1039/C8CY00304A
- W. Li, H. Wang, X. Jiang, J. Zhu, Z. Liu, X. Guo, C. Song, RSC Adv., 2018, 8, 7651–7669. DOI: 10.1039/C7RA13546G
- H. Yang, C. Zhang, P. Gao, H. Wang, X. Li, L. Zhong, W. Wei, Y. Sun, Catal. Sci. Technol., 2017, 7, 4580–4598. DOI: 10.1039/C7CY01403A
- W.-H. Wang, Y. Himeda, J. T. Muckerman, G. F. Manbeck, E. Fujita, Chem. Rev., 2015, 115, 12936–12973. DOI: 10.1021/acs.chemrev.5b00197
- Z. Li, Y. Qu, J. Wang, H. Liu, M. Li, S. Miao, C. Li, Joule, 2019, 3, 570–583. DOI: 10.1016/j.joule.2018.10.027
- Y. Ni, Z. Chen, Y. Fu, Y. Liu, W. Zhu, Z. Liu, Nat. Commun., 2018, 9, 3457. DOI: 10.1038/s41467-018-05880-4
- X. Zhang, A. Zhang, X. Jiang, J. Zhu, J. Liu, J. Li, G. Zhang, C. Song, X. Guo, J. CO2 Util., 2019, 29, 140–145. DOI: 10.1016/j.jcou.2018.12.002
- M. D. Porosoff, B. Yan, J. G. Chen, Energy Environ. Sci., 2016, 9, 62–73. DOI: 10.1039/C5EE02657A
- A. Álvarez, A. Bansode, A. Urakawa, A. V. Bavykina, T. A. Wezendonk, M. Makkee, J. Gascon, F. Kapteijn, Chem. Rev., 2017, 117, 9804–9838. DOI: 10.1021/acs.chemrev.6b00816
- A. Goeppert, M. Czaun, J.-P. Jones, G. K. Surya Prakash, G. A. Olah, Chem. Soc. Rev., 2014, 43, 7995–8048. DOI: 10.1039/C4CS00122B
- J. H. Kim, X. Ma, A. Zhou, C. Song, Catal. Today, 2006, 111, 74–83. DOI: 10.1016/j.cattod.2005.10.017
- H. Arakawa, M. Aresta, J. N. Armor, M. A. Barteau, E. J. Beckman, A. T. Bell, J. E. Bercaw, C. Creutz, E. Dinjus, D. A. Dixon, K. Domen, D. L. DuBois, J. Eckert, E. Fujita, D. H. Gibson, W. A. Goddard, D. W. Goodman, J. Keller, G. J. Kubas, H. H. Kung, J. E. Lyons, L. E. Manzer, T. J. Marks, K. Morokuma, K. M. Nicholas, R. Periana, L. Que, J. Rostrup-Nielson, W. M. H. Sachtler, L. D. Schmidt, A. Sen, G. A. Somorjai, P. C. Stair, B. R. Stults, W. Tumas, Chem. Rev., 2001, 101, 953–996. DOI: 10.1021/cr000018s
- G. Centi, S. Perathoner, Catal. Today, 2009, 148, 191–205. DOI: 10.1016/j.cattod.2009.07.075
- M. Mikkelsen, M. Jørgensen, F. C. Krebs, Energy Environ. Sci., 2010, 3, 43–81. DOI: 10.1039/B912904A
- K. I. Dement'ev, O. S. Dementeva, M. I. Ivantsov, M. V. Kulikova, M. V. Magomedova, A. L. Maximov, A. S. Lyadov, A. V. Starozhitskaya, M. V. Chudakova, Pet. Chem., 2022, 62, 445–474. DOI: 10.1134/S0965544122050012
- K. O. Kim, A. A. Shesterkina, M. A. Tedeeva, K. E. Kartavova, P. V. Pribytkov, S. F. Dunaev, A. L. Kustov, Russ. J. Phys. Chem. A, 2023, 97, 582–586. DOI: 10.1134/S0036024423040167
- A. Álvarez, A. Bansode, A. Urakawa, A. V. Bavykina, T. A. Wezendonk, M. Makkee, J. Gascon, F. Kapteijn, Chem. Rev., 2017, 117, 9804–9838. DOI: 10.1021/acs.chemrev.6b00816
- D. N. Gorbunov, M. V. Nenasheva, M. V. Terenina, Yu. S. Kardasheva, S. V. Kardashev, E. R. Naranov, A. L. Bugaev, A. V. Soldatov, A. L. Maximov, E. A. Karakhanov, Pet. Chem., 2022, 62, 1–39. DOI: 10.1134/S0965544122010054
- F. Studt, M. Behrens, E. L. Kunkes, N. Thomas, S. Zander, A. Tarasov, J. Schumann, E. Frei, J. B. Varley, F. Abild-Pedersen, J. K. Nørskov, R. Schlögl, ChemCatChem, 2015, 7, 1105–1111. DOI: 10.1002/cctc.201500123
- E. L. Kunkes, F. Studt, F. Abild-Pedersen, R. Schlögl, M. Behrens, J. Catal., 2015, 328, 43–48. DOI: 10.1016/j.jcat.2014.12.016
- M. B. Fichtl, D. Schlereth, N. Jacobsen, I. Kasatkin, J. Schumann, M. Behrens, R. Schlögl, O. Hinrichsen, Appl. Catal., A, 2015, 502, 262–270. DOI: 10.1016/j.apcata.2015.06.014
- J. Wang, G. Li, Z. Li, C. Tang, Z. Feng, H. An, H. Liu, T. Liu, C. Li, Sci. Adv., 2017, 3, e1701290. DOI: 10.1126/sciadv.1701290
- O. Martin, A. J. Martín, C. Mondelli, S. Mitchell, T. F. Segawa, R. Hauert, C. Drouilly, D. Curulla-Ferré, J. Pérez-Ramírez, Angew. Chem., Int. Ed., 2016, 55, 6261–6265. DOI: 10.1002/anie.201600943
- K. Sun, Z. Fan, J. Ye, J. Yan, Q. Ge, Y. Li, W. He, W. Yang, C.-j. Liu, J. CO2 Util., 2015, 12, 1–6. DOI: 10.1016/j.jcou.2015.09.002
- F. Studt, I. Sharafutdinov, F. Abild-Pedersen, C. F. Elkjær, J. S. Hummelshøj, S. Dahl, I. Chorkendorff, J. K. Nørskov, Nat. Chem., 2014, 6, 320–324. DOI: 10.1038/nchem.1873
- S. Gulati, S. Vijayan, Mansi, S. Kumar, B. Harikumar, M. Trivedi, R. S. Varma, Coord. Chem. Rev., 2023, 474, 214853. DOI: 10.1016/j.ccr.2022.214853
- S. Kuld, M. Thorhauge, H. Falsig, C. F. Elkjær, S. Helveg, I. B. Chorkendorff, J. Sehested, Science, 2016, 352, 969–974. DOI: 10.1126/science.aaf0718
- R. van den Berg, G. Prieto, G. Korpershoek, L. I. van der Wal, A. J. van Bunningen, S. Lægsgaard-Jørgensen, P. E. de Jongh, K. P. de Jong, Nat. Commun., 2016, 7, 13057. DOI: 10.1038/ncomms13057
- J. Szanyi, D. W. Goodman, Catal. Lett., 1991, 10, 383–390. DOI: 10.1007/BF00769173
- J. Yoshihara, S. C. Parker, A. Schafer, C. T. Campbell, Catal. Lett., 1995, 31, 313–324. DOI: 10.1007/BF00808595
- J. Yoshihara, C. T. Campbell, J. Catal., 1996, 161, 776–782. DOI: 10.1006/jcat.1996.0240
- R. M. Palomino, P. J. Ramírez, Z. Liu, R. Hamlyn, I. Waluyo, M. Mahapatra, I. Orozco, A. Hunt, J. P. Simonovis, S. D. Senanayake, J. A. Rodriguez, J. Phys. Chem. B, 2018, 122, 794–800. DOI: 10.1021/acs.jpcb.7b06901
- N. Pasupulety, A. A. Al-Zahrani, M. A. Daous, S. Podila, H. Driss, Arabian J. Chem., 2021, 14, 102951. DOI: 10.1016/j.arabjc.2020.102951
- A. Bansode, B. Tidona, P. R. von Rohr, A. Urakawa, Catal. Sci. Technol., 2013, 3, 767–778. DOI: 10.1039/C2CY20604H
- H. Ban, C. Li, K. Asami, K. Fujimoto, Catal. Commun., 2014, 54, 50–54. DOI: 10.1016/j.catcom.2014.05.014
- X. Jiang, X. Nie, X. Guo, C. Song, J. G. Chen, Chem. Rev., 2020, 120, 7984–8034. DOI: 10.1021/acs.chemrev.9b00723
- T. Phongamwong, U. Chantaprasertporn, T. Witoon, T. Numpilai, Y. Poo-arporn, W. Limphirat, W. Donphai, P. Dittanet, M. Chareonpanich, J. Limtrakul, Chem. Eng. J., 2017, 316, 692–703. DOI: 10.1016/j.cej.2017.02.010
- S. Kattel, P. J. Ramírez, J. G. Chen, J. A. Rodriguez, P. Liu, Science, 2017, 355, 1296–1299. DOI: 10.1126/science.aal3573
- X.-K. Wu, H.-M. Yan, W. Zhang, J. Zhang, G.-J. Xia, Y.-G. Wang, J. Energy Chem., 2021, 61, 582–593. DOI: 10.1016/j.jechem.2021.02.016
- P. Wu, B. Yang, ACS Catal., 2017, 7, 7187–7195. DOI: 10.1021/acscatal.7b01910
- J. Nakamura, I. Nakamura, T. Uchijima, Y. Kanai, T. Watanabe, M. Saito, T. Fujitani, J. Catal., 1996, 160, 65–75. DOI: 10.1006/jcat.1996.0124
- C. Shen, K. Sun, Z. Zhang, N. Rui, X. Jia, D. Mei, C.-j. Liu, ACS Catal., 2021, 11, 4036–4046. DOI: 10.1021/acscatal.0c05628
- M. S. Frei, C. Mondelli, A. Cesarini, F. Krumeich, R. Hauert, J. A. Stewart, D. Curulla Ferré, J. Pérez-Ramírez, ACS Catal., 2020, 10, 1133–1145. DOI: 10.1021/acscatal.9b03305
- K. Samson, M. Śliwa, R. P. Socha, K. Góra-Marek, D. Mucha, D. Rutkowska-Zbik, J.-F. Paul, M. Ruggiero-Mikołajczyk, R. Grabowski, J. Słoczyński, ACS Catal., 2014, 4, 3730–3741. DOI: 10.1021/cs500979c
- N. Rui, Z. Wang, K. Sun, J. Ye, Q. Ge, C.-j. Liu, Appl. Catal., B, 2017, 218, 488–497. DOI: 10.1016/j.apcatb.2017.06.069
- A. Cao, Z. Wang, H. Li, J. K. Nørskov, ACS Catal., 2021, 11, 1780–1786. DOI: 10.1021/acscatal.0c05046
- L. Yao, Y. Pan, D. Wu, J. Li, R. Xie, Z. Peng, Inorg. Chem. Front., 2021, 8, 2433–2441. DOI: 10.1039/D1QI00129A
- J. Ye, C. Liu, Q. Ge, J. Phys. Chem. C, 2012, 116, 7817–7825. DOI: 10.1021/jp3004773
- L. Yao, X. Shen, Y. Pan, Z. Peng, J. Catal., 2019, 372, 74–85. DOI: 10.1016/j.jcat.2019.02.021
- M. S. Frei, C. Mondelli, R. García-Muelas, K. S. Kley, B. Puértolas, N. López, O. V. Safonova, J. A. Stewart, D. Curulla Ferré, J. Pérez-Ramírez, Nat. Commun., 2019, 10, 3377. DOI: 10.1038/s41467-019-11349-9
- X. Jia, K. Sun, J. Wang, C. Shen, C.-j. Liu, J. Energy Chem., 2020, 50, 409–415. DOI: 10.1016/j.jechem.2020.03.083
- N. Rui, F. Zhang, K. Sun, Z. Liu, W. Xu, E. Stavitski, S. D. Senanayake, J. A. Rodriguez, C.-J. Liu, ACS Catal., 2020, 10, 11307–11317. DOI: 10.1021/acscatal.0c02120
- N. Yusuf, F. Almomani, Fuel, 2023, 332, 126027. DOI: 10.1016/j.fuel.2022.126027
- Y. Wang, S. Kattel, W. Gao, K. Li, P. Liu, J. G. Chen, H. Wang, Nat. Commun., 2019, 10, 1166. DOI: 10.1038/s41467-019-09072-6
- R. Singh, K. Tripathi, K. K. Pant, Fuel, 2021, 303, 121289. DOI: 10.1016/j.fuel.2021.121289
- J. Zhu, D. Ciolca, L. Liu, A. Parastaev, N. Kosinov, E. J. M. Hensen, ACS Catal., 2021, 11, 4880–4892. DOI: 10.1021/acscatal.1c00131
- A. Modak, A. Ghosh, A. Bhaumik, B. Chowdhury, Adv. Colloid Interface Sci., 2021, 290, 102349. DOI: 10.1016/j.cis.2020.102349
- M. Ren, Y. Zhang, X. Wang, H. Qiu, Catalysts, 2022, 12, 403. DOI: 10.3390/catal12040403
- F. Conrad, C. Massue, S. Kühl, E. Kunkes, F. Girgsdies, I. Kasatkin, B. Zhang, M. Friedrich, Y. Luo, M. Armbrüster, G. R. Patzke, M. Behrens, Nanoscale, 2012, 4, 2018–2028. DOI: 10.1039/C2NR11804A
- S. Huygh, A. Bogaerts, E. C. Neyts, J. Phys. Chem. C, 2016, 120, 21659–21669. DOI: 10.1021/acs.jpcc.6b07459
- H. Idriss, Chem Catal., 2022, 2, 1549–1560. DOI: 10.1016/j.checat.2022.05.022
- Y.-x. Pan, C.-j. Liu, D. Mei, Q. Ge, Langmuir, 2010, 26, 5551–5558. DOI: 10.1021/la903836v
- S. Kattel, B. Yan, J. G. Chen, P. Liu, J. Catal., 2016, 343, 115–126. DOI: 10.1016/j.jcat.2015.12.019
- N. Nomura, T. Tagawa, S. Goto, React. Kinet. Catal. Lett., 1998, 63, 21–25. DOI: 10.1007/BF02475425
- D. Laudenschleger, H. Ruland, M. Muhler, Nat. Commun., 2020, 11, 3898. DOI: 10.1038/s41467-020-17631-5
- T. Lunkenbein, J. Schumann, M. Behrens, R. Schlögl, M. G. Willinger, Angew. Chem., Int. Ed., 2015, 54, 4544–4548. DOI: 10.1002/anie.201411581
- T. Lunkenbein, F. Girgsdies, T. Kandemir, N. Thomas, M. Behrens, R. Schlögl, E. Frei, Angew. Chem., Int. Ed., 2016, 55, 12708–12712. DOI: 10.1002/anie.201603368
- B. Liang, J. Ma, X. Su, C. Yang, H. Duan, H. Zhou, S. Deng, L. Li, Y. Huang, Ind. Eng. Chem. Res., 2019, 58, 9030–9037. DOI: 10.1021/acs.iecr.9b01546
- A. Tsoukalou, P. M. Abdala, D. Stoian, X. Huang, M.-G. Willinger, A. Fedorov, C. R. Müller, J. Am. Chem. Soc., 2019, 141, 13497–13505. DOI: 10.1021/jacs.9b04873
- A. Pustovarenko, A. Dikhtiarenko, A. Bavykina, L. Gevers, A. Ramírez, A. Russkikh, S. Telalovic, A. Aguilar, J.-L. Hazemann, S. Ould-Chikh, J. Gascon, ACS Catal., 2020, 10, 5064–5076. DOI: 10.1021/acscatal.0c00449
- G. Noh, E. Lam, J. L. Alfke, K. Larmier, K. Searles, P. Wolf, C. Copéret, ChemSusChem, 2019, 12, 968–972. DOI: 10.1002/cssc.201900134
- J. Xiao, D. Mao, X. Guo, J. Yu, Appl. Surf. Sci., 2015, 338, 146–153. DOI: 10.1016/j.apsusc.2015.02.122
- G. Wang, D. Mao, X. Guo, J. Yu, Int. J. Hydrogen Energy, 2019, 44, 4197–4207. DOI: 10.1016/j.ijhydene.2018.12.131
- F. Lin, X. Jiang, N. Boreriboon, Z. Wang, C. Song, K. Cen, Appl. Catal., A, 2019, 585, 117210. DOI: 10.1016/j.apcata.2019.117210
- S. Bai, Q. Shao, Y. Feng, L. Bu, X. Huang, Small, 2017, 13, 1604311. DOI: 10.1002/smll.201604311
- Z. Shi, Q. Tan, D. Wu, AIChE J., 2019, 65, 1047–1058. DOI: 10.1002/aic.16490
- I. Sharafutdinov, C. F. Elkjær, H. W. Pereira de Carvalho, D. Gardini, G. L. Chiarello, C. D. Damsgaard, J. B. Wagner, J.-D. Grunwaldt, S. Dahl, I. Chorkendorff, J. Catal., 2014, 320, 77–88. DOI: 10.1016/j.jcat.2014.09.025
- C. Perego, P. Villa, Catal. Today, 1997, 34, 281–305. DOI: 10.1016/S0920-5861(96)00055-7
- M. Behrens, Catal. Today, 2015, 246, 46–54. DOI: 10.1016/j.cattod.2014.07.050
- R. van den Berg, J. Zečević, J. Sehested, S. Helveg, P. E. de Jongh, K. P. de Jong, Catal. Today, 2016, 272, 87–93. DOI: 10.1016/j.cattod.2015.08.052
- D. Chen, D. Mao, J. Xiao, X. Guo, J. Yu, J. Sol-Gel Sci. Technol., 2018, 86, 719–730. DOI: 10.1007/s10971-018-4680-4
- X. Dong, F. Li, N. Zhao, Y. Tan, J. Wang, F. Xiao, Chin. J. Catal., 2017, 38, 717–725. DOI: 10.1016/S1872-2067(17)62793-1
- W. Cai, P. R. de la Piscina, J. Toyir, N. Homs, Catal. Today, 2015, 242, 193–199. DOI: 10.1016/j.cattod.2014.06.012
- Z.-Q. Wang, Z.-N. Xu, S.-Y. Peng, M.-J. Zhang, G. Lu, Q.-S. Chen, Y. Chen, G.-C. Guo, ACS Catal., 2015, 5, 4255–4259. DOI: 10.1021/acscatal.5b00682
- Z.-Q. Wang, Z.-N. Xu, M.-J. Zhang, Q.-S. Chen, Y. Chen, G.-C. Guo, RSC Adv., 2016, 6, 25185–25190. DOI: 10.1039/C6RA02929A
- T. Fujitani, M. Saito, Y. Kanai, T. Watanabe, J. Nakamura, T. Uchijima, Appl. Catal., A, 1995, 125, L199–L202. DOI: 10.1016/0926-860X(95)00049-6
- M. Saito, T. Fujitani, M. Takeuchi, T. Watanabe, Appl. Catal., A, 1996, 138, 311–318. DOI: 10.1016/0926-860X(95)00305-3
- Q. Tan, Z. Shi, D. Wu, Ind. Eng. Chem. Res., 2018, 57, 10148–10158. DOI: 10.1021/acs.iecr.8b01246
- S. Tada, S. Satokawa, Catal. Commun., 2018, 113, 41–45. DOI: 10.1016/j.catcom.2018.05.009
- Y. Li, W. Na, H. Wang, W. Gao, J. Porous Mater., 2017, 24, 591–599. DOI: 10.1007/s10934-016-0295-8
- M. L. Poutsma, L. F. Elek, P. A. Ibarbia, A. P. Risch, J. A. Rabo, J. Catal., 1978, 52, 157–168. DOI: 10.1016/0021-9517(78)90131-8
- US Patent 4393144, 1983.
- Y. Matsumura, W.-J. Shen, Y. Ichihashi, M. Okumura, J. Catal., 2001, 197, 267–272. DOI: 10.1006/jcat.2000.3094
- S. Kattel, B. Yan, J. G. Chen, P. Liu, J. Catal., 2016, 343, 115–126. DOI: 10.1016/j.jcat.2015.12.019
- A. Gotti, R. Prins, J. Catal., 1998, 175, 302–311. DOI: 10.1006/jcat.1998.1996
- J. H. Kwak, L. Kovarik, J. Szanyi, ACS Catal., 2013, 3, 2094–2100. DOI: 10.1021/cs4001392
- N. Iwasa, H. Suzuki, M. Terashita, M. Arai, N. Takezawa, Catal. Lett., 2004, 96, 75–78. DOI: 10.1023/B:CATL.0000029533.41604.13
- X. Zhou, J. Qu, F. Xu, J. Hu, J. S. Foord, Z. Zeng, X. Hong, S. C. E. Tsang, Chem. Commun., 2013, 49, 1747–1749. DOI: 10.1039/C3CC38455A
- H. Bahruji, M. Bowker, G. Hutchings, N. Dimitratos, P. Wells, E. Gibson, W. Jones, C. Brookes, D. Morgan, G. Lalev, J. Catal., 2016, 343, 133–146. DOI: 10.1016/j.jcat.2016.03.017
- J. Xu, X. Su, X. Liu, X. Pan, G. Pei, Y. Huang, X. Wang, T. Zhang, H. Geng, Appl. Catal., A, 2016, 514, 51–59. DOI: 10.1016/j.apcata.2016.01.006
- Z. Li, J. Wang, Y. Qu, H. Liu, C. Tang, S. Miao, Z. Feng, H. An, C. Li, ACS Catal., 2017, 7, 8544–8548. DOI: 10.1021/acscatal.7b03251
- P. Gao, S. Dang, S. Li, X. Bu, Z. Liu, M. Qiu, C. Yang, H. Wang, L. Zhong, Y. Han, Q. Liu, W. Wei, Y. Sun, ACS Catal., 2018, 8, 571–578. DOI: 10.1021/acscatal.7b02649
- P. Gao, S. Li, X. Bu, S. Dang, Z. Liu, H. Wang, L. Zhong, M. Qiu, C. Yang, J. Cai, W. Wei, Y. Sun, Nat. Chem., 2017, 9, 1019–1024. DOI: 10.1038/nchem.2794
- P. Sharma, J. Sebastian, S. Ghosh, D. Creaser, L. Olsson, Catal. Sci. Technol., 2021, 11, 1665–1697. DOI: 10.1039/D0CY01913E
- N. Rui, Z. Wang, K. Sun, J. Ye, Q. Ge, C.-j. Liu, Appl. Catal., B, 2017, 218, 488–497. DOI: 10.1016/j.apcatb.2017.06.069
- J. L. Snider, V. Streibel, M. A. Hubert, T. S. Choksi, E. Valle, D. C. Upham, J. Schumann, M. S. Duyar, A. Gallo, F. Abild-Pedersen, T. F. Jaramillo, ACS Catal., 2019, 9, 3399–3412. DOI: 10.1021/acscatal.8b04848
- Y.-L. Men, Y. Liu, Q. Wang, Z.-H. Luo, S. Shao, Y.-B. Li, Y.-X. Pan, Chem. Eng. Sci., 2019, 200, 167–175. DOI: 10.1016/j.ces.2019.02.004
- C.-Y. Chou, R. F. Lobo, Appl. Catal., A, 2019, 583, 117144. DOI: 10.1016/j.apcata.2019.117144
- K. Kovnir, J. Osswald, M. Armbrüster, R. Giedigkeit, T. Ressler, Yu. Grin, R. Schlögl, Stud. Surf. Sci. Catal., 2006, 162, 481–488, DOI: 10.1016/S0167-2991(06)80943-2
- J. C. Bauer, X. Chen, Q. Liu, T.-H. Phan, R. E. Schaak, J. Mater. Chem., 2008, 18, 275–282. DOI: 10.1039/B712035D
- H. Choi, S. Oh, S. B. Trung Tran, J. Y. Park, J. Catal., 2019, 376, 68–76. DOI: 10.1016/j.jcat.2019.06.051
- S. E. Collins, J. J. Delgado, C. Mira, J. J. Calvino, S. Bernal, D. L. Chiavassa, M. A. Baltanás, A. L. Bonivardi, J. Catal., 2012, 292, 90–98. DOI: 10.1016/j.jcat.2012.05.005
- M. Ding, R. W. Flaig, H.-L. Jiang, O. M. Yaghi, Chem. Soc. Rev., 2019, 48, 2783–2828. DOI: 10.1039/C8CS00829A
- B. Liang, J. Ma, X. Su, C. Yang, H. Duan, H. Zhou, S. Deng, L. Li, Y. Huang, Ind. Eng. Chem. Res., 2019, 58, 9030–9037. DOI: 10.1021/acs.iecr.9b01546
- H. Noh, C.-W. Kung, T. Islamoglu, A. W. Peters, Y. Liao, P. Li, S. J. Garibay, X. Zhang, M. R. DeStefano, J. T. Hupp, O. K. Farha, Chem. Mater., 2018, 30, 2193–2197. DOI: 10.1021/acs.chemmater.8b00449
- B. An, J. Zhang, K. Cheng, P. Ji, C. Wang, W. Lin, J. Am. Chem. Soc., 2017, 139, 3834–3840. DOI: 10.1021/jacs.7b00058
- T. Liu, X. Hong, G. Liu, ACS Catal., 2020, 10, 93–102. DOI: 10.1021/acscatal.9b03738
- Y. Yin, B. Hu, X. Li, X. Zhou, X. Hong, G. Liu, Appl. Catal., B, 2018, 234, 143–152. DOI: 10.1016/j.apcatb.2018.04.024
- Y. Chen, H. Li, W. Zhao, W. Zhang, J. Li, W. Li, X. Zheng, W. Yan, W. Zhang, J. Zhu, R. Si, J. Zeng, Nat. Commun., 2019, 10, 1885. DOI: 10.1038/s41467-019-09918-z
- X. Li, G. Liu, D. Xu, X. Hong, S. C. Edman Tsang, J. Mater. Chem. A, 2019, 7, 23878–23885. DOI: 10.1039/C9TA03410B
- B. Hu, Y. Yin, Z. Zhong, D. Wu, G. Liu, X. Hong, Catal. Sci. Technol., 2019, 9, 2673–2681. DOI: 10.1039/C8CY02546K
- H. Kobayashi, J. M. Taylor, Y. Mitsuka, N. Ogiwara, T. Yamamoto, T. Toriyama, S. Matsumura, H. Kitagawa, Chem. Sci., 2019, 10, 3289–3294. DOI: 10.1039/C8SC05441J





