2021 Volume 4 Issue 5
|
INEOS OPEN, 2021, 4 (5), 189–194 Journal of Nesmeyanov Institute of Organoelement Compounds Download PDF |
|
Novel Lanthanide(III) Complexes with Pyrrolidinofullerene Ligands:
Synthesis and Characterization
a Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, Moscow, 119991 Russia
b Institute for Problems of Chemical Physics, Russian Academy of Sciences, pr. Akademika Semenova 1, Chernogolovka, Moscow Oblast, 142432 Russia
Corresponding author: E. A. Khakina, e-mail: khakina90@ineos.ac.ru
Received 12 November 2021; accepted 17 January 2022
Abstract

Novel europium and samarium complexes with pyrrolidinofullerene-based ligands are synthesized. Their compositions and molecular structures are elucidated using elemental analysis, MALDI-TOF mass spectrometry, as well as FTIR and NMR spectroscopy. It is shown that the metal ion coordinates with one pyrrolidinofullerene molecule. The photoluminescence spectra of the resulting complexes are similar to those of the free fullerene ligand.
Key words: pyrrolidinofullerenes, lanthanide(III) complexes, photoluminescence.
Introduction
Owing to their three-dimensional structure and unique electronic properties, fullerenes and their derivatives are useful in various applications, for example, as materials for organic electronic devices [1, 2] and in biomedicine [3–7].
Different approaches for the chemical modification of a fullerene core were developed [7–16] that pave the way to the design and synthesis of fullerene derivatives bearing a chelating group for further coordination with metal ions. The coordination chemistry of fullerenes is an actively growing research area [17]. The metal complexes with fullerene-containing ligands exhibit interesting properties owing to the intramolecular fullerene–metal interactions. The photoactive donor–acceptor fullerene–porphyrin dyads were suggested as the synthetic analogs of natural photosynthetic antennas [18, 19]. Recently, the spin-crossover properties were observed for the complexes of hexakis-substituted [60]fullerene adducts bearing 2,6-bis(pyrazol-1-yl)pyridine ligands coordinated with Fe(II) ions [20]. These results suggest that [60]fullerene can serve as an excellent scaffold for the design of new spin-switching materials.
Owing to intensive monochromatic photoluminescence, the complexes of rare-earth metals with organic ligands find application in materials for organic light-emitting diodes (OLEDs) and optical amplifiers [21]. However, there are very few complexes of fullerenes with rare-earth metals reported to date [17]. Ballesteros et al. [22] described the synthesis and photophysical properties of double-decker lanthanide(III) bis(phthalocyaninato)-C60 dyads.
We reported previously [23] the preparation of fullerene-containing Eu(III) complexes by mixing a fullerene derivative bearing chelating pyridinyl groups with a Eu(III) complex possessing three 4,4,4-trifluoro-1-(2-naphthyl)-1,3-butanedione ligands and two water molecules. The investigation of the photoluminescence properties of the resulting complexes showed that the fullerene ligand does not donate energy to the Eu(III) ion, whereas the emission of the rare-earth metal ion is quenched by it. This suggests that energy is transferred from the Eu(III) ion to the fullerene core. Similar quenching was observed when this type of complexes was mixed with a π-conjugated polymer [23], which indicates that fullerenes can act as electron acceptors.
Herein, we report the synthesis, characterization, and photoluminescence properties of novel Eu(III) and Sm(III) complexes bearing pyrrolidinofullerene ligands.
Results and discussion
Pyrrolidinofullerenes PyF-1 and PyF-2 were obtained by the [2+3]-cycloaddition of azomethine ylides with [60]fullerene (Scheme 1) [15]. The N-substituted derivatives of picolylamines were used as substrates for the generation of azomethine ylides as reported previously [16].

Scheme 1. Synthesis of pyrrolidinofullerene-based ligands PyF-1 and PyF-2.
The trans-arrangement of two 2-pyridyl groups in pyrrolidinofullerene PyF-1 was earlier confirmed by X-ray single-crystal diffraction analysis (Fig. 1) [16].

Figure 1. Structure of PyF-1 according to the X-ray single-crystal diffraction data. Hydrogen atoms and the disorder of some attached groups are not shown for clarity. Reproduced from P. A. Troshin, A. S. Peregudov, S. I. Troyanov, R. N. Lyubovskaya, Russ. Chem. Bull., 2008, 57, 887–912, DOI: 10.1007/s11172-008-0126-4 with permission of the authors.
The target complexes were synthesized in two steps (Scheme 2). First, Eu(III) and Sm(III) complexes bearing two coordinated water molecules and three 1,3-diketonate ligands (Eu-A and Sm-A) were prepared according to the published procedures [22]. Then, complexes Eu-A and Sm-A were introduced into the ligand exchange reactions with PyF-1 and PyF-2 to form complexes Eu-B, Eu-C, Sm-B, and Sm-C bearing the fullerene ligands.
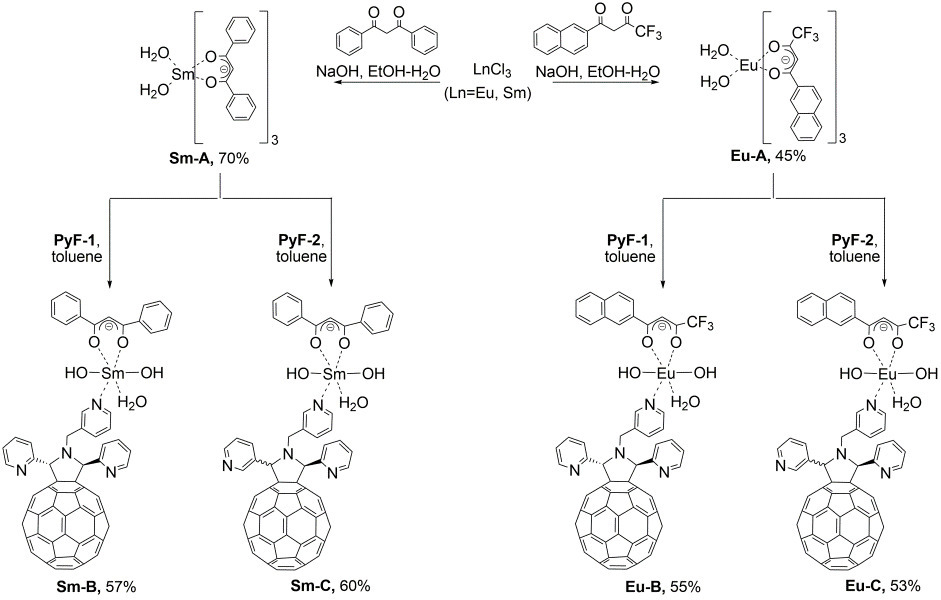
Scheme 2. Synthesis of the lanthanide(III) complexes with the pyrrolidinofullerene ligands.
The elemental analysis data obtained for fullerene-containing complexes Eu-B, Eu-C, Sm-B, and Sm-C indicate the presence of one pyrrolidinofullerene and one diketonate moiety in their molecules. Apparently, the metal ions in these complexes are coordinated with the nitrogen atoms of one of the pyridyl groups of the pyrrolidinofullerene ligand. To compensate for the charge of the lanthanide ions, the coordination of two hydroxy groups was proposed. To confirm this hypothesis, a series of spectral studies were performed. Unfortunately, we failed to obtain single crystals of the resulting complexes suitable for X-ray diffraction analysis. Therefore, the molecular structures of these compounds were assigned based only on the spectroscopic data.
The MALDI-TOF spectra of Eu-B showed a signal of the molecular ion for the expected complex composition (m/z = 1479) and signals of different fragment ions (Figs. S1, S2 in the Electronic supplementary information (ESI)). The mass spectrometric investigation of Eu-C, Sm-B, and Sm-C by either MALDI-TOF or ESI MS did not reveal any metal-containing ions: only the signals of PyFH+ ions (m/z = 1009) were registered for all the complexes explored. These results can be explained by the instability of these compounds under ionization conditions.
The FTIR spectra of complexes Eu-B, Eu-C, Sm-B, and Sm-C showed the characteristic absorption bands of pyrrolidinofullerene and diketonate units (Figs. S3, S4 in the ESI), which confirms the presence of both types of the ligands in the complexes obtained.
The investigation of rare-earth metal complexes by NMR spectroscopy is often complicated by the paramagnetic nature of some of the metal ions [21]. However, the spectral data for the samarium complexes appeared to be analyzable owing to the less marked broadening of the signals. For comparison, Fig. 2 depicts the 1H NMR spectra of ligand PyF-1 and complex Sm-B. The signals of all the protons in the spectrum of Sm-B are broadened compared to the corresponding signals of PyF-1 due to the paramagnetism of Sm(III) ions. Table 1 lists the chemical shifts and integral intensities for the representative sets of signals of PyF-1 and Sm-B. Almost all the chemical shifts for the complex and free pyrrolidinofullerene ligand are virtually identical, except for the chemical shifts of H4 and H6–H10 protons. These signals appeared to be upfield shifted by 0.01 ppm relative to the signals in the spectrum of PyF-1 due to the presence of the metal ions. Thus, the 1H NMR spectroscopic data confirm the structure suggested for Sm-B complex. The 13C NMR spectrum of Sm-B exhibited 48 signals, 4 in the region of sp3-carbon nuclei (C1, C2, C3, and C4) and 44 in the region of sp2-carbon nuclei (Fig. 3), which is fully consistent with the proposed structure of Sm-B. The 1H–13C HSQC NMR spectrum afforded unambiguous assignment of the signals of the sp3-carbon nuclei (Fig. 3).

Figure 2.1H NMR spectra of PyF-1 (bottom) and Sm-B (top) in CDCl3.

Figure 3. 1H–13C HSQC NMR spectrum of Sm-B in acetone-d6–CS2.
Table 1. Comparison of the chemical shifts and integral intensities for the signals in the 1H NMR spectra of PyF-1 [16, refined data] and Sm-B
|
Proton |
Chemical shift, ppm |
Integral intensity |
Multiplicity (J, Hz) |
|||
|
PyF-1 [16] |
Sm-B |
PyF-1 |
Sm-B |
PyF-1 |
Sm-B |
|
|
H1 |
3.78 |
3.78 |
1H |
1H |
d (14.2) |
d (14.2) |
|
H1 |
4.36 |
4.36 |
1H |
1H |
||
|
H2 |
8.86 |
8.86 |
2H |
2H |
br. s |
br. s |
|
H3 |
7.75 |
7.75 |
2H |
2H |
m |
m |
|
H4 |
7.30 |
7.29 |
2H |
2H |
m |
m |
|
H5 |
7.70 |
7.70 |
2H |
2H |
br. s |
br. s |
|
H6 |
7.98 |
7.99 (+H12) |
1H |
5H (+H12) |
d (7.7) |
m |
|
H7 |
7.43 |
7.42 |
1H |
1H |
dd (7.7, 4.7) |
m |
|
H8 |
8.64 |
8.63 |
1H |
1H |
dd (4.7, 1.6) |
d (4.8) |
|
H9 |
8.72 |
8.71 |
1H |
1H |
d (2.1) |
br.s. |
|
H10 |
6.81 |
6.80 |
2H |
2H |
br. s |
br.s. |
|
H11 |
– |
6.87 |
– |
1H |
– |
s |
|
H12 |
– |
7.99 (+H6) |
– |
5H (+H6) |
– |
m |
|
H13 |
– |
7.51 |
– |
4H |
– |
m |
|
H14 |
– |
7.55 |
– |
2H |
– |
m |
The analysis of the 1H NMR spectra of Eu(III) complexes Eu-B-C (Figs. S6, S7 in the ESI) is complicated due to the shifts and broadening of the signals induced by the paramagnetic nature of Eu(III) ions [23]. However, the number of the observed resonances in the 19F and 13C NMR spectra of complexes Eu-B-C (Figs. S7–S9 in the ESI) match the proposed structures of these compounds.
We investigated also the photoluminescence properties of complexes Eu-B, Eu-C, Sm-B, and Sm-C: the resulting spectra appeared to be very close to each other (Fig. 4). It is known that the broad emission band at 600–850 nm (±100 nm depending on the solvent) is characteristic of photoluminescence spectra of the fullerene derivatives [24–26]. The observed photoluminescence intensity is much lower than that of the related Eu(III) complex deprived of the fullerene ligand (Fig. 5). Hence, it can be concluded that the fullerene ligands quench the photoluminescence of Eu(III) ions. This is in good agreement with our previous findings for another type of complexes [24].

Figure 4. Photoluminescence spectra of complexes Eu-B, Sm-B, and Sm-C in chlorobenzene.

Figure 5. Photoluminescence spectrum of the fullerene-free Eu(III) complex in chlorobenzene (intensity divided by 3). Reproduced from A. Fuchsbauer, O. A. Troshina, P. A. Troshin, R. Koeppe, R. N. Lyubovskaya, N. S. Sariciftci, Adv. Funct. Mater., 2008, 18, 2808–2814, DOI: 10.1002/adfm.200800377 with permission of the authors.
Experimental
General remarks
All the solvents used for the synthesis and spectroscopic measurements were purified according to the standard procedures. Europium(III) chloride hexahydrate (EuCl3·6H2O, 99%, Sigma-Aldrich), samarium(III) chloride hexahydrate (SmCl3·6H2O, 99%, Sigma-Aldrich), 2-pyridinecarboxaldehyde (99%, Acros Organics), 3-pyridinecarboxaldehyde (98%, Acros Organics), 4,4,4-trifluoro-1-(2-naphthyl)-1,3-butanedione (99%, Sigma-Aldrich), 1,3-diphenyl-1,3-propanedione (98%, Sigma- Aldrich), and C60 fullerene (98%, Sigma-Aldrich) were used as received without further purification.
Pyrrolidinofullerenes PyF-1 and PyF-2, lanthanide(III) complexes Eu-A and Sm-A were synthesized according to the published procedures [15, 16, 22].
The proton, carbon, and fluorine nuclear magnetic resonance spectra were recorded with Bruker Avance 400 (operating at 400, 101, and 376 MHz for 1H, 13C, and 19F nuclei, respectively) and Bruker Avance 600 (operating at 600 and 150 MHz for 1H and 13C nuclei, respectively) NMR spectrometers. The chemical shifts (δ) for 1H nuclei are reported in ppm relative to the residual solvent signal (CDCl3 δ = 7.26 ppm, acetone-d6 δ = 2.05 ppm), those for 13C nuclei—relative to the deuterated solvent signal (CDCl3 δ = 77.16 ppm, acetone-d6 δ = 29.84 and 206.26 ppm), and those for 19F nuclei—relative to the external standard CFCl3 (δ = 0 ppm).
The FTIR spectra were recorded with a Spectrum BX-II Fourier spectrometer (Perkin Elmer) in KBr pellets.
The PL spectra were recorded using a M.U.T Tristan light fiber spectrometer. The excitation source was a Xe-lamp (900 W) equipped with a monochromator of the linewidth below 2 nm. All the solution measurements were performed in 1 mm special optical glass cuvettes from Hellma.
Syntheses
General procedure for the syntheses of lanthanide(III) complexes with pyrrolidinofullerene ligands Eu-B, Eu-C, Sm-B, and Sm-C. Pyrrolidinofullerene (0.050 mmol) and the corresponding lanthanide(III) diketonate complex (0.025 mmol) were dissolved in 50 mL of toluene in a round-bottom flask. The resulting mixture was refluxed for 10 min, then, cooled and concentrated under vacuum. The target product was precipitated by diethyl ether and isolated as an amorphous dark-brown solid by centrifugation.
Complex Eu-B. Yield: 55%.1Н NMR (600 MHz, acetone-d6): δ 3.76 (d, 1H, J = 10.0 Hz), 4.33 (d, 1H, J = 10.1 Hz), 6.75 (br. s, 2H), 7.24 (s, 2H), 7.37 (s, 1H), 7.71 (s, 4H), 8.09 (s, 1H), 8.8 (s, 3H) ppm. 13C{1Н} NMR (150 MHz, CS2–acetone-d6): δ 48.86, 74.23, 76.46, 122.82, 123.24, 124.69, 133.50, 135.92, 136.26, 136.52, 137.36, 139.43, 139.91, 141.47, 141.75, 141.84, 142.00, 142.07, 142.22, 142.47, 142.56, 143.05, 143.08, 144.49, 144.55, 145.05, 145.09, 145.16, 145.47, 145.59, 145.87, 145.91, 145.94, 146.14, 146.17, 146.45, 147.24, 149.86, 153.53, 155.96, 159.30 ppm. Anal. Calcd for C92H28N4O5EuF3: C, 74.75; H, 1.91; N, 3.79; F, 3.86. Found: C, 74.20; H, 1.83; N, 3.69; F, 3.50%.
Complex Eu-C. Yield: 53%. 1Н NMR (300 MHz, CDCl3): δ 3.85 (br. s, 1H), 4.42 (br. s, 1H), 6.04 (s, 1H), 7.45 (m, 4H), 7.81 (s, 2H), 8.52 (br. s, 1H), 8.80 (m, 3H), 9.13 (s, 1H) ppm. 13C{1Н} NMR (150 MHz, CS2–acetone-d6): δ 48.86, 73.31, 74.24, 74.99, 75.66, 122.82, 122.97, 123.26, 125.84, 128.36, 129.07, 133.11, 135.61, 135.98, 136.28, 136.54, 136.66, 137.68, 138.32, 139.44, 139.60, 139.65, 139.91, 140.19, 141.49, 141.61, 141.65, 141.71, 141.76, 141.83, 141.98, 142.03, 142.06, 142.09, 142.16, 142.23, 142.44, 142.47, 142.57, 142.61, 142.70, 143.03, 143.10, 143.19, 144.42, 144.50, 144.56, 144.61, 144.69, 145.08, 145.11, 145.16, 145.18, 145.32, 145.38, 145.49, 145.57, 145.70, 145.90, 145.93, 146.09, 146.12, 146.17, 146.28, 146.33, 146.67, 147.27, 147.36, 149.86, 150.13, 152.50, 153.65, 153.92, 157.14, 160.08 ppm. 19F NMR (376 MHz, acetone-d6): δ –80.11 (s).
Complex Sm-B. Yield: 57%. 1Н NMR (400 MHz, DMSO-d6): δ 3.78 (d, 1H, J = 14.2 Hz), 4.36 (d, 1H, J = 14.2 Hz), 6.79 (br. s, 2H), 6.87 (s, 1H), 7.29 (m, 4H), 7.42 (m, 1H), 7.49 (m, 3H), 7.54 (m, 2H), 7.68 (br. s, 2H), 7.75 (m, 2H), 8.00 (m, 4H), 8.62 (br. s, 1H), 8.71 (s, 1H), 8.86 (br. s, 2H) ppm. 13C{1Н} NMR (150 MHz, CS2–acetone-d6): δ 48.93, 74.40, 76.63, 93.07, 122.86, 123.49, 124.66, 127.40, 128.71, 128.99, 129.89, 132.33, 133.65, 134.42, 135.43, 136.29, 136.55, 139.51, 139.98, 141.56, 141.83, 141.91, 142.09, 142.17, 142.28, 142.56, 142.66, 143.14, 143.19, 144.58, 144.64, 145.14, 145.17, 145.25, 145.55, 145.66, 145.97, 146.01, 146.23, 146.26, 146.50, 147.33, 148.99, 149.91, 149.98, 153.57, 155.93, 159.33 ppm. Anal. Calcd for C93H31N4O5Sm: C, 77.86; H, 2.18; N, 3.91. Found: C, 78.01; H, 2.08; N, 3.55%.
Complex Sm-C. Yield: 60%. 1Н NMR (600 MHz, CDCl3): δ 3.75 (d, 1H, J = 14.1 Hz), 4.34 (d, 1H, J = 14.1 Hz), 5.96 (s, 1H), 6.89 (s, 1H), 7.22 (m, 1H), 7.32 (m, 1H), 7.38 (m, 1H), 7.44 (m, 3H), 7.52 (m, 2H), 7.58 (m, 1H), 7.75 (m, 2H), 8.01 (m, 3H), 8.41 (br. s, 1H), 8.63 (m, 1H), 8.67 (m, 2H), 8.75 (s, 1H), 8.88 (s, 1H), 9.08 (m, 1H), 9.24 (br. s, 1H) ppm. 13C{1Н} NMR (150 MHz, CS2–acetone-d6): δ 48.73, 73.24, 74.87, 75.61, 92.82, 122.86, 123.00, 123.38, 123.59, 125.81, 127.39, 128.69, 128.98, 129.86, 132.33, 133.33, 135.35, 135.58, 136.30, 136.47, 136.66, 137.69, 138.33, 139.64, 140.22, 141.66, 141.69, 141.76, 141.81, 141.87, 141.90, 142.03, 142.04, 142.08, 142.14, 142.22, 142.45, 142.53, 142.63, 142.68, 142.76, 143.09, 143.13, 143.18, 143.24, 144.46, 144.57, 144.66, 144.74, 145.13, 145.24, 145.38, 145.44, 145.61, 145.65, 145.73, 145.87, 145.95, 145.98, 146.03, 146.15, 146.18, 146.24, 146.32, 146.38, 146.64, 147.33, 147.42, 149.10, 149.95, 150.17, 151.14, 152.49, 153.6, 153.83, 157.11, 160.07 ppm.
Conclusions
The synthesis of four novel lanthanide(III) complexes bearing pyrrolidinofullerene-based ligands is presented. The compositions and molecular structures of these complexes were deduced from the elemental analysis, MALDI-TOF spectrometric, as well as IR and NMR spectroscopic data. In particular, it was shown that the metal ion coordinates with one pyrrolidinofullerene unit and one diketonate ligand, whereas the hydroxy groups and water molecules compensate for the ionic charge and saturate the coordination shell.
The investigation of the photoluminescence properties of the resulting fullerene-containing complexes showed that the fullerene ligands strongly quench emission of the lanthanide(III) ions. This quenching may be indicative of a photoinduced electron or energy transfer from the Ln(III) (Ln = Eu, Sm) ion to the fullerene core.
Acknowledgements
We are grateful to the Laboratory of Microanalysis of INEOS RAS for performing the elemental analyses and Dr. A. Fuchsbauer (JKU Linz) for measuring the PL spectra of the complexes.
The financial support was provided by the Ministry of Science and Higher Education of the Russian Federation (project no. 0089-2019-0010/AAAA-A19-119071190044-3). This work was carried out using the equipment of the Center for Molecular Composition Studies of INEOS RAS.
Electronic supplementary information
Electronic supplementary information (ESI) available online: mass spectrum of complex Eu-B; IR spectra of compounds PyF-1, Eu-A, Eu-B, Sm-A, and Sm-B; 1H and 13C{1H} NMR spectra of complexes Eu-B, Eu-C, Sm-B, and Sm-C; 19F NMR spectrum of compound Eu-C. For ESI, see DOI: 10.32931/io2121a.
References
- C.-Z. Li, H.-L. Yip, A. K.-Y. Jen, J. Mater. Chem., 2012, 22, 4161–4177. DOI: 10.1039/C2JM15126J
- P. A. Troshin, N. S. Sariciftci, in: Organic Nanomaterials: Synthesis, Characterization, and Device Applications, T. Torres, G. Bottari (Eds.), Wiley, Hoboken, 2013, ch. 25, pp. 549–578.
- S. Thakral, N. K. Thakral, in: Bio-Nanotechnology: A Revolution in Food, Biomedical and Health Sciences, D. Bagchi, M. Bagchi, H. Moriyama, F. Shahidi (Eds.), Blackwell, Oxford, 2013, ch. 24, pp. 424–442.
- Medicinal Chemistry and Pharmacological Potential of Fullerenes and Carbon Nanotubes, F. Cataldo, T. Da Ros (Eds.), Carbon Mater.: Chem. Phys., Springer, Dordrecht, 2008, vol. 1.
- A. Montellano, T. Da Ros, A. Bianco, M. Prato, Nanoscale, 2011, 3, 4035–4041. DOI: 10.1039/C1NR10783F
- M. A. Orlova, T. P. Trofimova, A. P. Orlov, O. A. Shatalov, J. Adv. Med. Med. Res., 2013, 3, 1731–1756.
- O. A. Kraevaya, A. S. Peregudov, I. A. Godovikov, E. V. Shchurik, V. M. Martynenko, A. F. Shestakov, J. Balzarini, D. Schols, P. A. Troshin, Chem. Commun., 2020, 56, 1179–1182. DOI: 10.1039/C9CC08400B
-
E. A. Khakina, P. A. Troshin, Russ. Chem. Rev., 2017, 86, 805–830. DOI: 10.1070/RCR4693
- H.-S. Lin, Y. Matsuo, Chem. Commun., 2018, 54, 11244–11259. DOI: 10.1039/C8CC05965A
- L. Đorđević, L. Casimiro, N. Demitri, M. Baroncini, S. Silvi, F. Arcudi, A. Credi, M. Prato, Angew. Chem., Int. Ed., 2021, 60, 313–320. DOI: 10.1002/anie.202009235
- M. R. Cerón, L. Echegoyen, J. Phys. Org. Chem., 2016, 29, 613–619. DOI: 10.1002/poc.3563
- N. Martín, M. Altable, S. Filippone, A. Martín-Domenech, Synlett, 2007, 20, 3077–3095. DOI: 10.1055/s-2007-990939
- Z.-Q. Liu, Curr. Org. Synth., 2017, 14, 999–1021. DOI: 10.2174/1570179414666161230152314
- S. Zhou, P. Trochimczyk, L. Sun, S. Hou, H. Li, Curr. Org. Chem., 2016, 20, 1490–1501. DOI: 10.2174/1385272820666151207194235
- P. A. Troshin, A. S. Peregudov, D. Mühlbacher, R. N. Lyubovskaya, Eur. J. Org. Chem., 2005, 3064–3074. DOI: 10.1002/ejoc.200500048
- P. A. Troshin, A. S. Peregudov, S. I. Troyanov, R. N. Lyubovskaya, Russ. Chem. Bull., 2008, 57, 887–912. DOI: 10.1007/s11172-008-0126-4
- M. A. Lebedeva, T. W. Chamberlain, A. N. Khlobystov, Chem. Rev., 2015, 115, 11301–11351. DOI: 10.1021/acs.chemrev.5b00005
- D. I. Schuster, K. Li, D. M. Guldi, C. R. Chim., 2006, 9, 892–908. DOI: 10.1016/j.crci.2005.11.013
- P. A. Troshin, R. Koeppe, A. S. Peregudov, S. M. Peregudova, M. Egginger, R. N. Lyubovskaya, N. S. Sariciftci, Chem. Mater., 2007, 19, 5363–5372. DOI: 10.1021/cm071243u
- M. Palacios-Corella, J. Ramos-Soriano, M. Souto, D. Ananias, J. Calbo, E. Ortí, B. M. Illescas, M. Clemente-León, N. Martín, E. Coronado, Chem. Sci., 2021, 12, 757–766. DOI: 10.1039/D0SC05875K
- T.-N. Trieu, T.-H. Dinh, H.-H. Nguyen, U. Abram, M.-H. Nguyen, Z. Anorg. Allg. Chem., 2015, 641, 1934–1940. DOI: 10.1002/zaac.201500158
- B. Ballesteros, G. de la Torre, A. Shearer, A. Hausmann, M. Á. Herranz, D. M. Guldi, T. Torres, Chem. Eur. J., 2010, 16, 114–125. DOI: 10.1002/chem.200902200
- K. P. Birin, Yu. G. Gorbunova, A. Yu. Tsivadze, Magn. Reson. Chem., 2010, 48, 505–515. DOI: 10.1002/mrc.2612
- A. Fuchsbauer, O. A. Troshina, P. A. Troshin, R. Koeppe, R. N. Lyubovskaya, N. S. Sariciftci, Adv. Funct. Mater., 2008, 18, 2808–2814. DOI: 10.1002/adfm.200800377
- D. Zhou, L. Gan, H. Tan, C. Luo, C. Huang, G. Yao, B. Zhang, J. Photochem. Photobiol., A, 1996, 99, 37–43. DOI: 10.1016/1010-6030(96)04386-9
- A. I. Kotelnikov, A. Yu. Rybkin, E. A. Khakina, A. B. Kornev, A. V. Barinov, N. S. Goryachev, A. V. Ivanchikhina, A. S. Peregudov, V. M. Martynenko, P. A. Troshin, Org. Biomol. Chem., 2013, 11, 4397–4404. DOI: 10.1039/C3OB40136G





