2021 Volume 4 Issue 3
|
INEOS OPEN, 2021, 4(3), 103–106 Journal of Nesmeyanov Institute of Organoelement Compounds |
|
Oligochitosan Hydrochloride: Preparation and Characterization
Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, Moscow, 119991 Russia
Corresponding author: E. A. Bezrodnykh, e-mail: bezrodnykh113@mail.ru
Received 18 March 2021; accepted 20 May 2021
Abstract

The methods commonly used for depolymerization of chitosan and preparation of oligochitosan are discussed. A synthetic approach to oligochitosan hydrochloride with a molecular weight below 16 kDa based on the treatment of parent industrial high molecular weight chitosan with a mixture of hydrochloric acid and hydrogen peroxide is described. A series of analytical protocols are used to determine the physicochemical properties and quality of resulting oligochitosan hydrochloride according to the European Pharmacopoeia 4.0.
Key words: chitosan, depolymerization, acid hydrolysis, hydrogen peroxide, oligochitosan.
Introduction
Since the discovery of penicillin by A. Fleming, dozens of antibiotics have been found out and introduced into medical practice. At the same time, bacteria, molds, and yeasts have become more resistant to classic antibiotics and more tolerant to natural and synthetic preservatives used in pharmaceutical, food, and cosmetic products. Nowadays, the misuse and overuse of antibiotics is a major global challenge. On the other side, the number of new antibiotics introduced into medical practice is constantly reducing. The World Health Organization has warned that the abuse of antibiotics risks taking the world back to the time when infections were incurable. In order to combat the growing threats from superbacteria and pathogenic fungi, all developed countries have planned a reduction in the use of antibiotics and rapid development of new antibiotics and synthetic chemicals that would display a wide range of antimicrobial activities [1].
Among the latter, chitosan is considered a nontoxic fungicide and bactericide for warm-blooded animals and plants. In 1983 the United States Food and Drug Administration allowed the use of chitosan as a food additive and the Environmental Protection Agency [2] authorized the use of chitosan in the agriculture and food industry. In the European Union, chitosan hydrochloride is approved for application in medical, food, and cosmetic industries [3].
Chitosan represents a polysaccharide that consists of glucosamine and N-acetylglucosamine. It is industrially manufactured by the deacetylation of crab/shrimp shell chitin, [(poly(N-acetylglucosamine)], in the presence of 30–50% sodium hydroxide solution at 70–130 °C for 1–8 h. The conditions for the chitin deacetylation determine the molecular weight (MW), degree of acetylation (DA) (or degree of deacetylation (DD)), polydispersity, solubility, and antimicrobial activity. An increase in the sodium concentration, process temperature, or duration may lead to a reduction in the MW, DA, solubility, and crystallinity. A recognition criterion between chitin and chitosan is the complete solubility of the product in an aqueous acidic media. Industrially produced chitosan usually features the following characteristics: DA = 4–25% (DD = 75–98%) and MW = 60–1500 kDa [4, 5].
The product is called chitosan if its MW is more than 16 kDa and oligochitosan if its MW is up to 16 kDa [6, 7].
There are many reports on the antimicrobial activity and safety of chitosan and its derivatives against bacteria and fungi. Nowadays, chitosan and oligochitosan are recognized as biocompatible, nontoxic to higher organisms antimicrobial polysaccharides that provide a synergistic or additive effect in combination with antibiotics [8–13].
Among the chemical methods commonly used for oligochitosan preparation, the main ones are acid hydrolysis, oxidative decomposition, and deamination. Although each method has its peculiarities, advantages, and drawbacks, the acid hydrolysis [14, 15] and application of hydrogen peroxide [16] seem preferable since they introduce lower chemical changes to the final structure of depolymerized chitosan [14, 17].
In this work, we describe the modified approach to the preparation of medical-grade oligochitosan hydrochloride that is suitable for application in pharmaceutical, food, and cosmetic products and compositions.
Results and discussion
Acid hydrolysis of chitosan
As it was shown, the rate of chitosan depolymerization under the action of hydrochloric acid depends on the DA and distribution of acetyl groups along chitosan polymer chains of parent chitosan, acid concentration, and temperature. The acid depolymerization of chitosan is very specific to the disruption of chitosan chain bonds. The rate of depolymerization reduces in the following order: between two N-acetylglucosamine (A–A) > N-acetylglucosamine–glucosamine (A–G or G–A) >> glucosamine–glucosamine (G–G) fragments. Partial deacetylation also occurs; therefore, the DA of oligochitosan obtained by acid hydrolysis is lower than that of starting chitosan. The rates of depolymerization and deacetylation depend on the acid concentration: thus, the rate of depolymerization with 12M HCl acid is about an order of magnitude higher than that of chitosan N-dеaсеtylation. Otherwise, the rate of depolymerization in 1–6 M HCl is equal to that of deacetylation [18].
From the practical point of view, the depolymerization of chitosan with 1M HCl seems to be an attractive method since it does not affect the chemical structure of oligochitosan although it represents a longer-lasting process than that in the case of the application of the concentrated acid.
As is shown in Fig. 1a, the MW of the parent chitosan (CHI‑p) with Mw = 350 kDa and DA = 12% rapidly declines at the process beginning but then the hydrolysis rate significantly reduces. This can be attributed to a faster disruption of glycoside bonds between two conjunct N-acetylglucosamine (A–A) groups and a further slower disruption of residual A–G and G–G fragments.

Figure 1. Effect of 1M HCl (a), 1.5% H2O2 in 1% acetic acid (b), and 1M HCl/1.5% H2O2 (c)
on the weight average molecular weight of chitosan depending on the reaction time (t).
Chitosan depolymerization with hydrogen peroxide
Similar to the hydrolysis in the acid medium, the rate and depth of chitosan depolymerization under the action of hydrogen peroxide depend on the chitosan MW, DA, distribution of acetyl groups along chitosan polymer chains, hydrogen peroxide concentration, solution pH, and temperature. The mechanism of depolymerization involves the cleavage of glycoside bonds between non-protonated G–A and G–G fragments which concentration grow with an increase in the solution pH [19].
Figure 1b demonstrates that the depolymerization rate is very high at the first reaction stage but reduces as soon as most of the G–A fragments are consumed. As a result, the deeper depolymerization of chitosan with hydrogen peroxide represents a long-lasting process at the low hydrogen peroxide concentration and requires a higher hydrogen peroxide concentration and higher process temperature. The latter parameters appear to be crucial for the product quality since the treatment of chitosan with hydrogen peroxide results in both a reduction in the MW and oxidation of the polymer chain backbone. These changes in the chemical structure and browning greatly intensify with a decrease in the product MW [20].
Preparation of oligochitosan by the modified method
The joint use of hydrochloric acid and hydrogen peroxide (Fig. 1c) stipulates a much faster reduction in the chitosan molecular weight and affords oligochitosan with MW < 16 kDa and very low solution viscosity (Fig. 2).

Figure 2. Kinematic viscosity of a 1% oligochitosan solution depending on the molecular weight.
The application of a mixture of hydrochloric acid and hydrogen peroxide furnishes the target product in high yields (70–90%) and allows for only minimal chemical changes in the oligochitosan structure. Furthermore, the resulting samples feature narrow dispersity (Mw/Mn = 1.2–1.6) and reduced content of acetylated units. The proposed method takes 40–60 min and affords oligochitosan hydrochloride with the quality corresponding to the EU Pharmacopoeia requirements (Table 1).
Table 1. Quality characteristics of oligochitosan hydrochloride compared with the requirements of EP 4.0 for chitosan hydrochloride
|
Characteristics |
EP 4.0 acceptable criteria for chitosan hydrochloride |
Actual data for oligochitosan hydrochloride |
|
Appearance |
white or almost white |
from snow white to chalky white |
|
Solubility in water |
soluble |
soluble |
|
Matter insoluble in water, % |
≤0.5% |
≤0.02% |
|
Color of 1% solution (brownish-yellow standards) |
≤ standard solution BY5 |
≤ standard solution BY6 |
|
Appearance of a solution |
opalescence ≤ the standard reference suspension II |
opalescence ≤ the standard reference suspension I |
|
Viscosity of 1% solution in water |
not specified (80–120% of the value stated on the label) |
1.03–1.20 cSt |
|
Solution pH |
4.0–6.0 |
2.6–2.8 |
|
Degree of acetylation, % |
unstandardized value |
1–2% |
|
Chlorides, % |
10.0–20.0% |
16.0–16.5% |
|
Heavy metals |
|
Fe < 15 ppm; |
|
Loss on drying (100–105 °C), % |
≤10% |
≤10% |
|
Sulfated ash, % |
≤1% |
≤0.01% |
Characterization of oligochitosan hydrochloride
Oligochitosan hydrochloride with MW = 10 kDa and reduced DA value as a consequence of the partial acid deacetylation of CHI-p was characterized according to the prescriptions of EP 2004 (Table 1). Its chemical structure is depicted in Fig. 3.
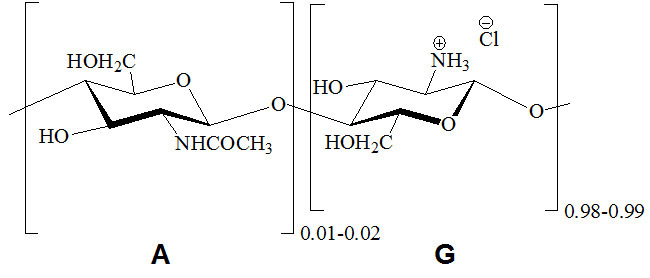
Figure 3. Chemical structure of oligochitosan hydrochloride.
Following the protocol, resulting chitosan hydrochloride was characterized by the appearance of 1% solution, solubility in water, solution pH, degree of acetylation, loss on drying, and the contents of chlorides, sulfated ash, and heavy metals.
As it follows from Table 1, the quality characteristics of resulting oligochitosan hydrochloride are equal to the requirements or better than those required by EP 4.0 with the only exception: 1% oligochitosan solution has pH values ranging within 2.6–2.8. This contradiction arises from the uncertainty of the required content of chlorides in chitosan hydrochloride. As is shown in Fig. 4, the value of pH of a 1% chitosan hydrochloride solution strongly depends on the molar ratio of glucosamine and hydrogen hydrochloride.

Figure 4. Dependence of pH of a 1% chitosan (DD = 98%) solution on the hydrochloric acid/chitosan amino group molar ratios.
The content of chlorides (16.0–16.5%) found in oligochitosan hydrochloride is equal to 0.96–0.98 molar ratio of HCl/glucosamine ratio and corresponds to the chitosan solution pH equal to 2.6–2.8 as is shown in Fig. 4.
Experimental
Materials
Chitosan (CHI-p) with MW = 350 kDa and DA = 12% was purchased from BIOPROGRESS (Russia). Hydrochloric acid (HCl) of analytical grade and 30% hydrogen peroxide (H2O2) were purchased from Merck.
Methods
General procedure for the hydrolysis of a 10% solution of chitosan (MW = 350 kDa, DA = 12%) in 1M hydrochloric acid. 10 g of chitosan was loaded into a 150 mL flask equipped with a stirrer and a backflow condenser and placed on a water bath heated to 70 °C. Then, 100 mL of a 1% (weight/volume) acetic acid solution or a mixture of 100 mL of 1M hydrochloric acid and 5 mL of 30 % hydrogen peroxide was added under stirring.
The degree of deacetylation (DD, mol %) was determined by 1H NMR spectroscopy [14] using the equipment of the Center for Molecular Composition Studies of INEOS RAS.
The apparent weight average (Mw) and number average (Mn) molecular weights (MWs) of the resulting samples of oligochitosan hydrochlorides were determined by high performance size exclusion chromatography according to the published procedure [16].
The kinematic viscosity of 1% aqueous solutions of oligochitosan was defined using a capillary viscometer (diameter 0.56 mm, viscometer constant 0.0093109 mm2/s2). Each solution was filtered through a Millipore filter with a pore diameter of 1 mm to remove impurities. The measurements were carried out at 25 ± 0.5 °С.
The content of chlorides in oligochitosan hydrochloride was measured at the Laboratory for Microanalysis of INEOS RAS.
The quality of oligochitosan hydrochloride was determined according to the prescriptions of EP 2004 [3].
Conclusions
The preparation of medical-grade oligochitosan hydrochloride is a complicated process and requires specific conditions that imply joint application of hydrochloric acid and hydrogen peroxide in order to avoid undesirable effects on the quality of a final product. Oligochitosan hydrochloride with the quality that meets the requirements of European Pharmacopoeia can be obtained upon precise selection of parent chitosan and under strict control of the reaction parameters. It should be noted that in order to prepare medical-grade oligochitosan, the precise conditions for depolymerization of initial chitosan should be selected depending on its specific molecular weight and acetylation degree.
Acknowledgements
This work was performed with financial support from the Ministry of Science and Higher Education of the Russian Federation using the equipment of the Center for Molecular Composition Studies of INEOS RAS.
References
- S. B. Zaman, M. A. Hussain, R. Nye, V. Mehta, K. T. Mamun, N. Hossain, Cureus, 2017, 9, e1403. DOI: 10.7759/cureus.1403
- The United States Pharmacopeia, Chitosan, USP 34–NF 29, Second Supplement, The United States Pharmacopeial Convention, 2011, Rockville, pp. 5361–5365.
- European Pharmacopoeia, 5th ed. (European Treaty Series, no. 50, vols. 1 and 2), 2004, Strasbourg, Directorate for the Quality of Medicines of the Ph. Eur., pp. 1248–1249.
- M. Rinaudo, Prog. Polym. Sci., 2006, 31, 603–632. DOI: 10.1016/j.progpolymsci.2006.06.001
- A. Muxika, A. Etxabide, J. Uranga, P. Guerrero, K. de la Caba, Int. J. Biol. Macromol., 2017, 105, 1358–1368. DOI: 10.1016/j.ijbiomac.2017.07.087
- S. N. Kulikov, S. A. Lisovskaya, P. V. Zelenikhin, E. A. Bezrodnykh, D. R. Shakirova, I. V. Blagodatskikh, V. E. Tikhonov, Eur. J. Med. Chem., 2014, 74, 169–178. DOI: 10.1016/j.ejmech.2013.12.017
- A. Verlee, S. Mincke, C. V. Stevens, Carbohydr. Polym., 2017, 164, 268–283. DOI: 10.1016/j.carbpol.2017.02.001
- P. Zou, X. Yang, J. Wang, Y. Li, H. Yu, Y. Zhang, G. Liu, Food Chem., 2016, 190, 1174–1181. DOI: 10.1016/j.foodchem.2015.06.076
- Z. Shariatinia, Adv. Colloid Interface Sci., 2019, 263, 131–194. DOI: 10.1016/j.cis.2018.11.008
- W.-H. Lo, F.-S. Deng, C.-J. Chang, C.-H. Lind, Molecules, 2020, 25, 5114. DOI: 10.3390/molecules25215114
- D.-S. Lee, Y.-M. Kim, M.-S. Lee, C.-B. Ahn, W.-K. Jung, J.-Y. Je, Bioorg. Med. Chem. Lett., 2010, 20, 975–978. DOI: 10.1016/j.bmcl.2009.12.049
- H. Mu, A. Zhang, L. Zhang, H. Niu, J. Duan, Food Control, 2014, 38, 215–220. DOI: 10.1016/j.foodcont.2013.10.032
- S. Kulikov, V. Tikhonov, I. Blagodatskikh, E. Bezrodnykh, S. Lopatin, R. Khairullin, Y. Philippova, S. Abramchuk, Carbohydr. Polym., 2012, 87, 545–550. DOI: 10.1016/j.carbpol.2011.08.017
- N. D. Al-Jbour, M. D. H. Beg, I. Gimbun, A. K. M. M. Alam, Int. J. Appl. Pharm., 2021, 13, 153–164. DOI: 10.22159/ijap.2021v13i2.32229
- C. Q. Qin, Y. M. Du, L. Xiao, Polym. Degrad. Stab., 2002, 76, 211–218. DOI: 10.1016/S0141-3910(02)00016-2
- E. A. Bezrodnykh, I. V. Blagodatskikh, S. N. Kulikov, P. V. Zelenikhin, I. A. Yamskov, V. E. Tikhonov, Carbohydr. Polym., 2018, 195, 551–557. DOI: 10.1016/j.carbpol.2018.05.007
- I. Aranaz, N. Acosta, C. Civera, B. Elorza. J. Mingo, C. Castro, M. De los Llanos Gandía, A. Heras Caballero, Polymers, 2018, 10, 213. DOI: 10.3390/polym10020213
- K. M. Vårum, M. H. Ottøy, O. Smidstød, Carbohydr. Polym., 2001, 46, 89–98. DOI: 10.1016/S0144-8617(00)00288-5
- F. Tian, Y. Liu, K. Hu, B. Zhao, Carbohydr. Polym., 2004, 57, 31–37. DOI: 10.1016/j.carbpol.2004.03.016
- A. Hirai, H. Odani, A. Nakajima, Polym Bull., 1991, 26, 87–94. DOI: 10.1007/BF00299352





