2021 Volume 4 Issue 1
|
INEOS OPEN, 2021, 4 (1), 24–28 Journal of Nesmeyanov Institute of Organoelement Compounds |
|
Synthesis and Reductive Amination of 1-Aryl-5-ferrocenyl-1H-pyrazole-4-carbaldehydes
Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, Moscow, 119991 Russia
Corresponding author: A. A. Simenel, e-mail: alexsim@ineos.ac.ru
Received 10 February 2021; accepted 1 April 2021
Abstract
An efficient method for the synthesis of 1-phenyl-5-ferrocenyl-1H-pyrazole-4-carbaldehyde is developed. Its reactivity in reductive amination is studied towards various amines.
Key words: ferrocene, formylpyrazoles, reductive amination.
Introduction
It is known that the substitution of aromatic cores in organic compounds for ferrocene gives rise to the products that feature the properties not typical for the initial compounds or less expressed [1, 2]. This is often associated with the unusual pharmacokinetics and biodegradation of ferrocene derivatives in the body. Nowadays, this approach is actively exploited in a search for new drugs. Many compounds that are currently under clinical trials have been developed as the derivatives of the well-known drugs with the established biological activity, for example, ferrocifen (an analog of tamoxifen) and ferroquine (an analog of chloroquine) [3]. The modification of various heterocyclic derivatives, including natural ones, with ferrocene is a popular line of research [4–6].
Pyrazole derivatives are widely used in catalysis, agricultural chemistry, and medicine, as well as building blocks for the synthesis of new compounds. The attractiveness of this heterocycle and its derivatives lies in their availability owing to a variety of methods for the formation of a pyrazole ring, which opens the way to the synthesis of a range of new analogs, including isomeric ones, bearing different substituents and modification of the properties of target compounds [7].
Until recently, it has been assumed that the compounds containing a pyrazole ring do not occur in nature [8, 9]. At the same time, it is well known that pyrazole derivatives exhibit a broad spectrum of biological activity, in particular, owing to their resemblance to other natural heterocyclic compounds. Pyrazole is included as a pharmacophore in a variety of biologically active synthetic derivatives (Fig. 1) [10, 11].

Figure 1
Special attention is drawn to the creation of new ferrocene-containing pyrazoles since the pyrazole derivatives have been shown to interact with enzymes and receptors of epidermal growth factor (EGFR, Aurora kinase, cyclooxygenase, etc.) [10, 12, 13]. Therefore, these compounds may display antitumor activity.
The introduction of a ferrocene moiety into a pyrazole pharmacophore ranks among the most important strategies for the creation of new drugs [10–14]. The antitumor potential of pyrazole derivatives was demonstrated in MCF-7 and MDA-MB-231 cancer cell lines [12]. Ferrocenylpyrazoles were also shown to suppress cell viability and induce apoptosis or necrosis in human breast cancer cells with low cytotoxicity towards healthy mammary epithelial cells [13]. Some ferrocenylpyrazole-containing chiral aminoethanol derivatives exhibited high activity against A549 and H322 cancer cell lines [15]. Ferrocenylpyrazole amines demonstrated non-selective and high activity against some of the most resistant pathogenic bacteria, namely, B. subtilis, Enterococcus sp., and P. aeruginosa, while the conventional drugs did not afford appreciable effects. These results encourage the use ferrocenylpyrazoles to combat the antibiotic-resistant strains of microorganisms [16, 17].

Figure 2
The biological studies revealed the effect of ferrocene-containing esters of amino acids bearing pyrazole linkers on the synaptic plasticity in the CA1 brain region of the hippocampus [18, 19].
Of particular interest is the design of ferrocenylpyrazole derivatives containing both the ferrocene residue and a functional, for example, aldehyde group in the pyrazole ring, which can be modified depending on the task [9, 10, 20]. The development of synthetic routes to such isomeric compounds enables variation of a mutual arrangement of the substituents in the heterocyclic ring as well as elucidation of the structure–activity relationships during biological screening.
The most convenient method for the synthesis of ferrocenylformylpyrazoles is the interaction of in situ generated acetylferrocene hydrazones with the Vilsmeier complex [21, 22, 23]. Thus, 1-aryl-3-ferrocenyl-4-pyrazolecarbaldehydes appeared to be convenient building blocks for the synthesis of Schiff bases with various amines and amino acids, unsaturated acids by the Perkin reaction, thioazolidinones, and the corresponding amines through the reductive amination [16, 17, 21–25].
Therefore, the development of synthetic routes to ferrocene–pyrazole derivatives bearing various functional groups is an urgent task. This work describes an approach to 1-aryl-5-ferrocenyl-1H-pyrazole-4-carbaldehydes and their reactivity in reductive amination.
Results and discussion
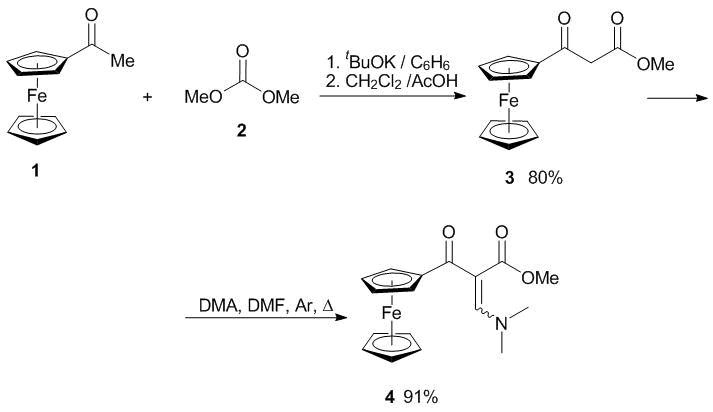
The Claisen condensation of acetylferrocene 1 with dimethyl carbonate 2 led to methyl ester of ferrocenoylacetic acid 3 when t-BuOK was used as a base in benzene (Scheme 1). The use of potassium tert-butoxide in a non-polar solvent significantly enhanced the yield of the target product owing to easy purification of the insoluble potassium salt of the resulting ketoester. Unlike ferrocenoylpyruvic esters, ferrocenoylacetic esters exist in chloroform predominantly in keto forms, as evidenced by the 1H and 13C NMR spectroscopic data. The 1H NMR spectrum shows a signal of the methylene protons at 3.77 ppm with the intensity of 2H, whereas the signal of the carbonyl group carbon nucleus in the 13C NMR spectrum appears at 195.9 ppm [26]. To obtain a synthetic equivalent of formylated ferrocenoylacetic acid ester 4, we used N,N-dimethylformamide dimethyl acetal. The reaction product was obtained in 91% yield (Scheme 1). It was shown that the best yield can be achieved when the reaction is carried out in N,N-dimethylformamide dimethyl acetal without an additional solvent upon heating in an inert atmosphere.
Scheme 1
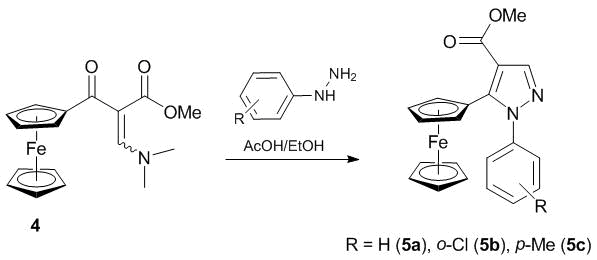
Resulting enamine derivative 4 was reacted with substituted phenylhydrazines in boiling ethanol. It should be noted that the reactions afforded only one possible isomer of compounds 5 (Scheme 2). The nature and position of a substituent in the phenylhydrazine did not affect the yields of the target products (78–82%). The latter crystallized from the reaction mixtures upon cooling and did not require further purification.
Scheme 2
The reduction of methyl esters of ferrocenylpyrazolecarboxylic acids 5 to the corresponding alcohols 6 was carried out under the action of lithium aluminum hydride in a mixture of THF and 1,4-dioxane. The subsequent oxidation of alcohols 6 to aldehydes 7 was accomplished upon treatment with manganese dioxide in dichloromethane at room temperature. As we have shown earlier [26], this reagent is the best one for the oxidation of ferrocenylpyrazole carbinols to aldehydes. The total yield in the two stages was ~90% (Scheme 3).

Scheme 3
Reductive amination
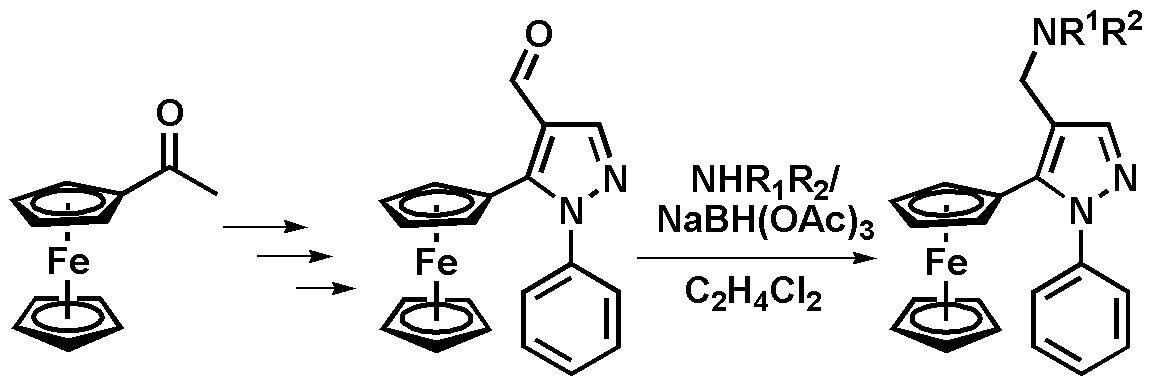
The reaction of carbonyl compounds, aldehydes and ketones, with ammonia, primary and secondary amines in the presence of reducing agents that lead to the formation of primary, secondary, and tertiary amines, respectively, is known as the reductive amination of carbonyl compounds or reductive alkylation of amines and is widely used in organic synthesis. It can be carried out in two versions: direct and stepwise. In the former case, the carbonyl compound and the amine are mixed with a suitable reducing agent without isolating an intermediate imine or iminium salt; in the latter case, the reaction includes the formation of the intermediate imine and its subsequent reduction [27]. Earlier our research group conducted a detailed study of the reductive amination of some ferrocenylpyrazolecarbaldehydes with 5- (p-aminophenyl)-10,15,20-tetraphenylporphyrin [28].
The investigation of the direct reductive amination of 1-phenyl-5-ferrocenyl-1H-pyrazole-4-carbaldehyde with various primary and secondary aliphatic and aromatic amines allowed us to reveal the main regularities of the process (Scheme 4). It was shown that the best yields can be achieved when sodium triacetoxyborohydride is used as a reducing agent upon refluxing in dichloroethane. When sodium borohydride was applied, the aldehyde group was reduced without the formation of an amine. The exploration of the interaction with primary and secondary aliphatic amines, as well as with aniline revealed that the best yields are obtained in the case of secondary cyclic amines.

Scheme 4
Experimental
General remarks
Acetylferrocene, sodium triacetoxyborohydride, lithium aluminum hydride, dimethyl carbonate, N,N-dimethylformamide dimethyl acetal, potassium tert-butoxide, and the amines explored were purchased from Acros Organics. The solvents were purified according to the standard procedures prior to the syntheses.
The electron ionization mass spectra were registered on a Finnigan Polaris Q instrument; the temperature of an ionization chamber was 250 °C; the energy of ionizing electrons was 70 eV. The 1H and 13C NMR spectra were recorded on a Bruker DRX-300 or Bruker DRX-500 spectrometer with the operating frequencies of 300 or 500 MHz and 75 or 125 MHz for 1H and 13C nuclei, respectively, at 30 °C in CDCl3. The chemical shifts are presented in δ (ppm) relative to the residual or deuterated solvent signals (7.26 ppm for 1H, 77.16 ppm for 13C). The melting points were determined on a Bibby Scientific SMP30 capillary melting point apparatus. The purity of the compounds was monitored by TLC on Silufol UV 254 plates. The chromatographic separation of the compounds was carried out using Kieselgel 0.035–0.070 mm, 90Å (Acros, Belgium).
Syntheses
Methyl 3-oxo-3-ferrocenylpropanoate (3). Acetylferrocene (22.8 g, 0.10 mol) was added to a suspension of t-BuOK (13.2 g, 0.11 mol) in benzene (150 mL). Then, methyl carbonic acid (0.11 mol) was added dropwise to the resulting suspension for 30 min. The stirred reaction mixture was refluxed for 6 h. After cooling to room temperature, a precipitate of the potassium salt was filtered off, washed with Et2O (300 mL), suspended in CH2Cl2 (150 mL), and acidified with acetic acid to pH = 5. The resulting mixture was washed with saturated aq. NaHCO3 (100 mL) and water (100 mL). The organic layer was separated and dried over Na2SO4. The solvent was removed under reduced pressure to give the target compounds as red crystals. Yield: 80%. Mp: 79–80 °С. Mass spectrum, m/z (Ire (%)): 286 [M]+ (100), 254 [M-CH3OH]+ (28), 213 [FcCO]+(40), 185 [Fc]+ (17). 1H NMR (CDCl3, 300 MHz, δ, ppm): 3.77 (s, 2H, CH2), 3.79 (s, 3H, CH3), 4.27 (s, 5H, Fc), 4.58 (s, 2H, Fc), 4.81 (s, 2H, Fc). 13C NMR (CDCl3, 75 MHz, δ, ppm): 46.7, 52.4, 69.7, 70.1, 73.0, 78.2, 168.0, 195.9.
Methyl 2-ferrocenoyl-3-dimethylamino acrylate (4). A stirred solution of ferrocenoylacetic acid ester 3 (0.10 mol) in N,N-dimethylformamide dimethyl acetal (2.7 mL, 0.20 mol) was refluxed in an argon atmosphere for 8 h. An excess of N,N-dimethylformamide dimethyl acetal was removed under reduced pressure. The resulting residue was purified by column chromatography on silica gel (eluent: PhH) to give compound 4 as red crystals. Yield: 91%. Mp: 111–112 °С. Mass spectrum, m/z (Ire (%)): 341 [M]+ (100), 276 [M-C5H5]+ (23). 1H NMR (CDCl3, 300 MHz, δ, ppm): 2.92 (br. s, 6H, CH3), 3.53 (s, 3H, OCH3), 4.19 (s, 5H, Fc), 4.46 and 4.56 (both s, 2H, Fc), 7.55 (s, 1H, CH). 13C NMR (CDCl3, 75 MHz, δ, ppm): 46.6, 52.9, 70.0, 71.4, 84.2, 101.0, 153.4, 168.7, 197.6.
General procedure for the synthesis of 1-aryl-5-ferrocenyl-1H-pyrazole-4-carbaldehydes. The corresponding arylhydrazine (5.1 mmol) was added to a solution of 2-ferrocenoyl-3-dimethylamino-acrylate methyl ester (5.0 mmol) in ethanol (20 mL) under an argon atmosphere. The resulting mixture was stirred at reflux for 4 h. The reaction progress was monitored by TLC. After cooling to 0 °C, the precipitate formed was filtered off, washed with cold dry acetone (10 mL), and dried over Na2SO4 to give the target compounds as orange crystals (5a), deep orange (5b) or deep purple (5c) powder.
Methyl 5-ferrocenyl-1-phenyl-1H-pyrazole-4-carboxylate (5a). Yield: 82%. Mp: 155–157 °C. Anal. Calcd for C21H18FeN2О3: C, 65.04; Н, 4.73; N, 7.06. Found: С, 65.31; Н, 4.70; N, 7.25%. Mass spectrum, m/z (Ire (%)): 386 [M]+ (100), 321 [M-Cp]+ (98), 291 [M-Cp-CH2O]+ (50). 1Н NMR (CDCl3, 300 MHz, δ, ppm.): 3.92 (s, 3H, CH3), 4.04 (s, 5H, Fc), 4.23 (s, 2H, Fc), 4.53 (s, 2H, Fc), 7.28 (m, 2H, Ph), 7.38 (m, 3H, Ph), 8.09 (s, 1H, Pz). 13C NMR (CDCl3, 75 MHz, δ, ppm.): 51.3, 68.8, 69.9, 71.1, 72.6, 112.4, 126.3, 128.3, 128.8, 140.4, 143.0, 144.6, 163.2.
Methyl 5-ferrocenyl-1-(p-tolyl)-1H-pyrazole-4-carboxylate (5b). Yield: 80%. Mp: 161 °C. Mass spectrum, m/z (Ire (%)): 400 [M]+ (67), 335 [M-Cp]+ (98), 305 [M-Cp-CH2O]+ (47). 1Н NMR (CDCl3, 500 MHz, δ, ppm.): 2.40 (s, 3H, CH3), 3.92 (s, 3H, OCH3), 4.04 (s, 5H, Fc), 4.23 (s, 2H, Fc), 4.54 (s, 2H, Fc), 7.16–7.19 (m, 4H, Ph), 8.07 (s, 1H, Pz). 13C NMR (CDCl3, 125 MHz, δ, ppm.): 21.1, 51.3, 68.7, 69.9, 71.1, 72.6, 112.1, 126.1, 129.3, 138.0, 138.4, 142.9, 144.5, 163.3.
Methyl 5-ferrocenyl-1-(2-chlorophenyl)-1H-pyrazole-4-carboxylate (5c). Yield: 78%. Mp: 124–126 °C. Mass spectrum, m/z (Ire (%)): 420 [M]+ (68), 355 [M-Cp]+ (33). 1Н NMR (CDCl3, 300 MHz, δ, ppm.): 3.93 (s, 3H, CH3), 4.06 (s, 5H, Fc), 4.22 (m, 2H, Fc), 4.48 (m, 1H, Fc), 4.58 (m, 1H, Fc), 7.34–7.47 (m, 3H, Ar), 7.53 (d, 1H, Ar, J = 7.7), 8.15 (s, 1H, Pz). 13C NMR (CDCl3, 125 MHz, δ, ppm.): 51.3, 68.9, 70.0, 71.2, 72.5, 113.9, 127.5, 130.0, 130.3, 131.1, 133.1, 137.9, 144.8, 145.4, 162.5.
General procedure for the reduction of ferrocenylpyrazolecarboxylic acid methyl esters. A solution of the corresponding ferrocenylpyrazolecarboxylic acid ester (5 mmol) in THF (50 mL) was added dropwise to a stirred suspension of lithium aluminum hydride (0.2 g, 5 mmol) in absolute 1,4-dioxane (50 mL). The resulting mixture was refluxed for 1 h. After cooling to room temperature, a saturated aqueous solution of NaCl (0.5 mL) was added. The resulting precipitate was filtered off and washed with Et2O (50 mL). The solution obtained was dried over anhydrous Na2SO4 and concentrated under reduced pressure on a rotary evaporator to give the target compounds as yellow crystals (6a) or orange oil (6b,c).
(5-Ferrocenyl-1-phenyl-1H-pyrazol-4-yl)methanol (6a). Yield: 95%. Mp: 148–149 °C. Anal. Calcd for C20H18FeN2О: С, 67.19; Н, 4.98; N, 7.69; Fe, 15.56. Found: С, 67.06; Н, 5.06; N, 7.82; Fe, 15.59%. Mass spectrum, m/z (Ire (%)): 358 [M]+ (100), 293 [M-Cp]+ (24). 1Н NMR (CDCl3, 300 MHz, δ, ppm): 1.82 (t, 1H, OH, J = 4.5), 4.07 (s, 5H, Fc), 4.22 and 4.24 (both t, 2H, Fc, J = 1.7), 4.94 (d, 2H, CH2, J = 4.5 Hz), 7.29–7.31 (m, 2H, Ph), 7.39–7.43 (m, 3H, Ph), 7.69 (s, 1H, Pz). 13C NMR (CDCl3, 75 MHz, δ, ppm.): 56.2, 68.6, 69.2, 69.6, 74.1, 119.5, 126.5, 128.2, 128.8, 139.3, 140.8, 140.9.
(5-Ferrocenyl-1-(p-tolyl)-1H-pyrazol-4-yl)methanol (6b). Yield: 93%. Mass spectrum, m/z (Ire (%)): 372 [M]+ (100), 370 [M-2H]+ (27), 352 [M-CH2O]+ (5), 307 [M-Cp]+ (8). 1Н (CDCl3, 300 MHz, δ, ppm): 2.39 (s, 3H, CH3), 3.39 (br. s, 1H, OH), 4.05 (s, 5H, Fc), 4.13 (s, 2H, Fc), 4.16 (s, 2H, Fc), 4.71 (s, 2H, CH2), 7.18 (d, 2H, Ar, J = 8.5 Hz), 7.30 (d, 2H, Ar, J = 8.4 Hz), 7.70 (s, 1H, Pz). 13C NMR (CDCl3, 75 MHz, δ, ppm.): 21.1, 58.7, 68.5, 69.7, 74.6, 104.7, 126.0, 129.3, 137.7, 138.1, 142.8, 152.6.
(5-Ferrocenyl-1-(2-chlorophenyl)-1H-pyrazol-4-yl)methanol (6c). Yield: 93%. Mass spectrum, m/z (Ire (%)): 392 [M]+ (100), 390 [M-2H]+ (20), 327 [M-Cp]+ (8). 1Н NMR (CDCl3, 300 MHz, δ, ppm): 3.32 (br. s, 1H, OH), 4.02 (s, 5H, Fc), 4.03 (s, 2H, Fc), 4.11 (s, 2H, Fc), 4.64 (s, 2H, CH2), 7.29–7.41 (m, 3H, Ar), 7.49 (d, 2H, Ar, J = 7.5 Hz), 7.72 (s, 1H, Pz). 13C NMR (CDCl3, 75 MHz, δ, ppm.): 58.5, 67.4, 68.8, 69.8, 73.4, 103.3, 127.5, 130.1, 130.2, 130.7, 133.0, 138.1, 144.5, 153.6.
General procedure for the synthesis of 5-ferrocenyl-1-aryl-1H-pyrazole-4-carbaldehydes. A solution of the corresponding ferrocenylpyrazolylmethanol (2 mmol ) in CH2Cl2 (10 mL) was added to a vigorously stirred suspension of activated MnO2 (0.87 g, 10 mmol) in dry CH2Cl2 (10 mL). The resulting suspension was stirred for 3 h at room temperature. The reaction mixture was filtered through celite. The residue obtained was washed with Et2O. The combined organic fractions were evaporated under reduced pressure and dried over anhydrous Na2SO4 to give the target compounds as oranges powders.
5-Ferrocenyl-1-phenyl-1H-pyrazole-4-carbaldehyde (7a). Yield: 94%. Mp: 137–138 °C. Anal. Calcd for C20H16FeN2О: С, 67.54; Н, 4.59; N, 7.77; Fe, 15.51. Found: С, 67.44; Н, 4.53; N, 7.86; Fe, 15.68%. Mass spectrum, m/z (Ire (%)): 356 [M]+ (97), 291 [M-Cp]+ (100). 1Н NMR (CDCl3, 500 MHz, δ, ppm.): 4.10 (s, 5H, Fc), 4.31 (s, 2H, Fc), 4.35 (s, 2H, Fc), 7.29 (m, 2H, Ar), 7.42 (m, 3H, Ar), 8.16 (s, 1H, Pz), 10.60 (s, 1H, CHO). 13C NMR (CDCl3, 75 MHz, δ, ppm.): 69.5, 70.3, 70.7, 71.5, 121.5, 126.2, 128.8, 129.0, 139.6, 141.5, 146.3, 185.2.
5-Ferrocenyl-1-(p-tolyl)-1H-pyrazole-4-carbaldehyde (7b). Yield: 90%. Mp: 140–141 °C. Anal. Calcd for C21H18FeN2О: С, 68.13; Н, 4.90; N, 7.57; Fe, 15.20. Found: С, 68.19; Н, 4.91; N, 7.44; Fe, 15.08%. Mass spectrum, m/z (Ire (%)): 370 [M]+ (100), 305 [M-Cp]+ (12). 1Н NMR (CDCl3, 500 MHz, δ, ppm.): 2.44 (s, 3H, CH3), 4.08 (s, 5H, Fc), 4.16 (s, 2H, Fc), 4.22 (s, 2H, Fc), 7.27 (m, 4H, Ar), 8.12 (s, 1H, Pz), 10.63 (s, 1H, CHO). 13C NMR (CDCl3, 75 MHz, δ, ppm.): 21.2, 68.7, 69.0, 69.9, 73.3, 104.9, 125.8, 129.6, 137.1, 139.2, 144.6, 151.3, 187.1.
5-Ferrocenyl-1-(2-chlorophenyl)-1H-pyrazole-4-carbaldehyde (7c). Yield: 87%. Mp: 156–157 °C. Anal. Calcd for C20H15ClFeN2О: C, 61.49; Н, 3.87; N, 7.17; Fe, 14.3. Found (%): С, 61.41; Н, 3.91; N, 7.14; Fe, 14.1%. Mass spectrum, m/z (Ire (%)): 390 [M]+ (100), 325 [M-Cp]+ (18). 1Н NMR (CDCl3, 500 MHz, δ, ppm.): 4.10 (s, 5H, Fc), 4.12 (s, 2H, Fc), 4.18 (s, 2H, Fc), 7.27–7.49 (m, 4H, Ar), 8.14 (s, 1H, Pz), 10.61 (s, 1H, CHO). 13C NMR (CDCl3, 75 MHz, δ, ppm.): 67.7, 69.0, 69.7, 73.3, 105.9, 125.8, 129.4, 129.6, 130.7, 133.1, 138.1, 144.6, 153.3, 189.1.
General procedure for the reductive amination. Sodium triacetoxyborohydride (0.3 g, 1.4 mmol) was added to a solution of ferrocenylpyrazolecarbaldehyde (1 mmol) and the corresponding amine (1.2 mmol) in dry 1,2-dichloroethane (35 mL). The resulting mixture was refluxed for 1–3 hours. After cooling to room temperature, a saturated aqueous solution of NaHCO3 (30 mL) was added. The target product was extracted with CH2Cl2 (2 × 30 mL). The combined organic fractions were washed with brine (30 mL), dried over anhydrous Na2SO4, and evaporated to dryness. The resulting residue was purified by column chromatography on silica gel (eluent: CHCl3–MeOH, 9: 1) to give the desired compounds as yellow (8a) or red (8b,c) crystals, or an orange oil (8d).
4-(n-Propylaminomethyl)-5-ferrocenyl-1-phenyl-1H-pyrazole (8a). Yield: 84%. Mp: 137 °C. Mass spectrum, m/z (Ire (%)): 399 [M]+ (100), 341 [M-(C3H7NH)]+ (15). 1Н NMR (CDCl3, 300 MHz, δ, ppm): 0.99 (t, 3H, CH3, J = 7.1), 1.61–1.74 (m, 2H, CH2), 2.08 (t, 2H, CH2, J = 7.1), 4.06 (s, 5H, Fc), 4.08 (s, 2H), 4.21 (m, 4H, Fc+CH2), 7.28–7.39 (m, 5H, Ar), 7.70 (s, 1H, Pz). 13C NMR (CDCl3, 75 MHz, δ, ppm): 11.9, 23.1, 44.2, 51.8, 68.2, 68.9, 69.4, 74.6, 118.6, 126.2, 127.7, 128.6, 137.9, 140.7, 140.8.
4-(1-Morpholinylmethyl)-5-ferrocenyl-1-phenyl-1H-pyrazole (8b). Yield: 91%. Mp: 180–181 °C. Anal. Calcd for C24H25FeN3О: C, 67.46; H, 5.90; N, 9.83. Found: С, 67.40; Н, 5.91; N, 9.86%. Mass spectrum, m/z (Ire (%)): 427 [M]+ (100), 341 [M-C4H8NO]+ (38). 1Н NMR (CDCl3, 300 MHz, δ, ppm): 2.64 (m, 4H, 2CH2), 3.72 (s, 2H, CH2), 3.80 (m, 4H, 2CH2), 4.05 (s, 5H, Fc), 4.21 and 4.35 (both m, 2H, Fc), 7.29–7.41 (m, 5H, Ar), 7.59 (s, 1H, Pz). 13C NMR (CDCl3, 75 MHz, δ, ppm): 53.6, 53.8, 67.2, 68.4, 69.2, 69.4, 74.8, 116.2, 126.5, 128.0, 128.8, 139.2, 141.0, 142.2.
4-(1-Pyrrolidinylmethyl)-5-ferrocenyl-1-phenyl-1H-pyrazole (8c). Yield: 93%. Mp: 117 °C. Anal. Calcd for C24H25FeN3: C, 70.08; H, 6.13; N, 10.22. Found: С, 70.19; Н, 6.18; N, 10.12%. Mass spectrum, m/z (Ire (%)): 411 [M]+ (100), 340 [M-C4H9N]+ (43). 1Н NMR (CDCl3, 300 MHz, δ, ppm): 1.87 (m, 4H, CH2), 2.71 (m, 4H, CH2), 3.88 (s, 2H, CH2), 4.06 (s, 5H, Fc), 4.10 and 4.14 (both t, 2H, Fc, J = 1.8), 7.28–7.40 (m, 5H, Ar), 7.63 (s, 1H, Pz). 13C NMR (CDCl3, 75 MHz, δ, ppm): 23.5, 50.5, 54.3, 68.1, 69.1, 69.3, 74.8, 118.0, 126.3, 127.6, 128.6, 138.2, 140.8, 141.4.
4-(Phenylaminomethyl)-5-ferrocenyl-1-phenyl-1H-pyrazole (8d). Yield: 87%. Anal. Calcd for C26H23FeN3: C, 72.07; H, 5.35; N, 9.70. Found: С, 72.03; Н, 5.33; N, 9.60%. Mass spectrum, m/z (Ire (%)): 433 [M]+ (52). 1H NMR (CDCl3, 300 MHz, δ, ppm): 4.06 (s, 5H, Fc), 4.19 (m, 4H, Fc+CH2), 4.52 (s, 2H, Fc), 6.80–6.83 (m, 3H, Ar), 7.27–7.47 (m, 7H, Ar), 7.71 (s, 1H, Pz). 13C NMR (CDCl3, 75 MHz, δ, ppm): 39.2, 68.5, 68.9, 69.5, 70.0, 70.3, 74.0, 112.7, 117.6, 120.7, 126.3, 128.0, 128.7, 129.3, 138.8, 139.7, 141.0, 148.0.
Conclusions
A convenient synthetic approach to 1-aryl-5-ferrocenyl-1H-pyrazole-4-carbaldehydes was developed starting from acetylferrocene as a key precursor. The total yield of the target product in five stages reached 54%. The reductive amination of 1-phenyl-5-ferrocenyl-1H-pyrazole-4-carbaldehydes with various amines was studied.
Acknowledgements
This work was carried out with financial support from the Ministry of Science and Higher Education of the Russian Federation using the equipment of the Center for Molecular Composition Studies of INEOS RAS.
References
- L. V. Snegur, A. A. Simenel, A. N. Rodionov, V. I. Boev, Russ. Chem. Bull., 2014, 63, 26–36. DOI: 10.1007/s11172-014-0390-4
- V. N. Babin, Yu. A. Belousov, V. I. Borisov, V. V. Gumenyuk, Yu. S. Nekrasov, L. A. Ostrovskaya, I. K. Sviridova, N. S. Sergeeva, A. A. Simenel, L. V. Snegur, Russ. Chem. Bull., 2014, 63, 2405–2422. DOI: 10.1007/s11172-014-0756-7
- Bioorganometallics: Biomolecules, Labeling, Medicine, G. Jaouen (Ed.), Wiley, Weinheim, 2006.
- C. Ornelas, New J. Chem., 2011, 35, 1973–1985. DOI: 10.1039/C1NJ20172G
- D. R. van Staveren, N. Metzler-Nolte, Chem. Rev., 2004, 104, 5931–5986. DOI: 10.1021/cr0101510
- M.-G. A. Shvekhgeimer, Russ. Chem. Rev., 1996, 65, 41–80. DOI: 10.1070/RC1996v065n01ABEH000199
- P. S. Parameswaran, C. G. Naik, V. R. Hegde, J. Nat. Prod., 1997, 60, 802–803. DOI: 10.1021/np970134z
- The Chemistry of Heterocyclic Chemistry:Pyrazoles, Pyrazolines, Pyrazolidines, Indazoles and Condensed Rings, R. H. Wiley, L. C. Behr, R. Fusco, C. H. Jarboe (Eds.), John Wiley & Sons Ltd, 1967, pp. 3–20.
- Comprehensive Heterocyclic Chemistry III, A. R. Katritzky, C. Ramsden, E. Scriven, R. Taylor (Eds.), Elsevier, Oxford, 2008, vol. 4.
- K. Karrouchi, S. Radi, Y. Ramli, J. Taoufik, Y. N. Mabkhot, F. A. Al-aizari, M. Ansar, Molecules, 2018, 23, 134. DOI: 10.3390/molecules23010134
- G. N. Lipunova, E. V. Nosova, V. N. Charushin, O. N. Chupakhin, J. Fluorine Chem., 2015, 175, 84–109. DOI: 10.1016/j.jfluchem.2015.03.011
- F. K. Keter, J. Darkwa, BioMetals, 2012, 25, 9–21. DOI: 10.1007/s10534-011-9496-4
- R. Wang, H. Chen, W. Yan, M. Zheng, T. Zhang, Y, Zhang, Eur. J. Med. Chem., 2020, 190, 112109. DOI: 10.1016/j.ejmech.2020.112109
- A. S. Hassan, T. S. Hafez, J. Appl. Pharm. Sci., 2018, 8, 156–165. DOI: 10.7324/JAPS.2018.8522
- S.-L. Shen, J. Zhu, M. Li, B.-X. Zhao, J.-Y. Miao, Eur. J. Med. Chem., 2012, 54, 287–294. DOI: 10.1016/j.ejmech.2012.05.008
- I. Damljanović, M. Vukićević, N. Radulović, R. Palić, E. Ellmerer, Z. Ratković, M. D. Joksović, R. D. Vukićević, Bioorg. Med. Chem. Lett., 2009, 19, 1093–1096. DOI: 10.1016/j.bmcl.2009.01.006
- I. Damljanović, M. Čolović, M. Vukićević, D. Manojlović, N. Radulović, K. Wurst, G. Laus, Z. Ratković, M. Joksović, R. D. Vukićević, J. Organomet. Chem., 2009, 694, 1575–1580. DOI: 10.1016/j.jorganchem.2009.01.045
- A. N. Rodionov, L. V. Snegur, Y. V. Dobryakova, M. M. Ilyin Jr, V. A. Markevich, A. A. Simenel., Appl. Organomet. Chem., 2020, 34, e5276. DOI: 10.1002/aoc.5276
- A. N. Rodionov, L. V. Snegur, A. A. Simenel, Yu. V. Dobryakova, V. A. Markevich, Russ. Chem. Bull., 2017, 66, 136–142. DOI: 10.1007/s11172-017-1711-1
- B. F. Abdel-Wahab, R. E. Khidre, A. A. Farahat, ARKIVOC, 2011, 196–245. DOI: 10.3998/ark.5550190.0012.103
- M. Joksović, Z. Ratković, M. Vukićević, R. D. Vukićević, Synlett, 2006, 16, 2581–2584. DOI: 10.1055/s-2006-950436
- E. Yu. Osipova, A. A. Simenel, A. A. Rodionov, V. V. Kachala, K. Ya. Zherebker, MITHT Bull., 2009, 4 (2), 100–105.
- A. N. Rodionov, A. A. Simenel, Yu. S. Nekrasov, V. V. Kachala, E. Yu. Osipova, K. Ya. Zherebker, Russ. Chem. Bull., 2010, 59, 405–410. DOI: 10.1007/s11172-010-0093-4
- A. Pejović, A. Minić, J. Bugarinović, M. Pešić, I. Damljanović, D. Stevanovića, V. Mihailović, J. Katanić, G. A. Bogdanović, Polyhedron, 2018, 155, 382–389. DOI: 10.1016/j.poly.2018.08.071
- M. D. Joksović, V. Marković, Z. D. Juranić, T. Stanojković, L. S. Jovanović, I. S. Damljanović, K. M. Szécsényi, N. Todorović, S. Trifunović, R. D. Vukićević, J. Organomet. Chem., 2009, 694, 3935–3942. DOI: 10.1016/j.jorganchem.2009.08.013
- A. N. Rodionov, A. A. Simenel, A. A. Korlyukov, V. V. Kachala, S. M. Peregudova, K. Ya. Zherebker, E. Yu. Osipova, J. Organomet. Chem., 2011, 696, 2108–2115. DOI: 10.1016/j.jorganchem.2010.11.018
- A. F. Abdel-Magid, K. G. Carson, B. D. Harris, C. A. Maryanoff, R. D. Shah, J. Org. Chem., 1996, 61, 3849–3862. DOI: 10.1021/jo960057x
- E. Yu. Osipova, A. N. Rodionov, A. A. Simenel, Yu. A. Belousov, O. M. Nikitin, V. V. Kachala, J. Porphyrins Phthalocyanines, 2012, 16, 1225–1232. DOI: 10.1142/S1088424612501246





