2020 Volume 3 Issue 3
|
|
INEOS OPEN, 2020, 3 (3), 109–111 Journal of Nesmeyanov Institute of Organoelement Compounds Download PDF
|
|
Zinc-Containing Composite Based on a Hypercrosslinked Polymer Sorbent
in the Sorption of Toxic and Foul-Smelling Substances
Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, Moscow, 119991 Russia
Corresponding author: S. E. Lyubimov, e-mail: lssp452@mail.ru
Received 18 June 2020; accepted 8 July 2020
Abstract
A facile technique for introducing zinc oxide into a highly porous polystyrene matrix is proposed. The resulting composite sorbent can efficiently remove hydrogen sulfide, foul-smelling and toxic products of degradation of natural remains (putrescine, cadaverine, indole, and skatole), as well as organic solvents such as methanol and benzene from the air and, thus, holds great promise for potential application in environmental protection.
Key words: hypercrosslinked polystyrene, sorption, zinc oxide, hydrogen sulfide, ptomaine.
Introduction
One of the most important global environmental problems is the air pollution. The danger is caused by the release of various volatile and toxic substances which change the air composition and quality. The latter may have negative impacts not only on the public health but also on the planet's climate [1]. The environmental experts divide all the factors that affect the air into anthropogenic and natural. The greatest damage to environment comes from the former—the factors that are associated with human activity [2]. The major contributors to the air pollution are industrial productions, transport, and landfills [3]. Of particular concern for population are the substances that possess, besides toxic effect, also foul odor, especially in the case of a law odor threshold. These compounds include hydrogen sulfide and volatile components of ptomaine, namely, cadaverine (1,5-diaminopentane), putrescine (1,4‑diaminobutane), indole, and skatole (3-methylindole). Of potential hazard are also low-boiling organic solvents. This is connected not only with their flammability but, to a greater extent, with their toxicity. The volatile toxic components are removed predominantly by sorption methods. The vapor sorption processes consist in a diffusion transition of a gaseous component into the sorbent pores and its retention on the active regions of the carrier. The sorbents are usually different synthetic and natural porous materials: activated carbons, silica gels, aluminum oxide, and high-molecular compounds [4]. The most important characteristics of the sorbents are porosity, pore sizes and structure, chemical composition, sorption capacity, and specific surface area. One of the promising sorbents successfully produced on an industrial scale is hypercrosslinked polystyrene. This is stipulated by the extremely high specific surface area of hypercrosslinked polystyrene (up to 1500 m2/g), which enables the sorption of a range of organic and inorganic compounds from aqueous, organic and air media [5, 6]. The pore sizes in hypercrosslinked polymer sorbents (3–80 nm on average) allow for the production of hybrid organic–inorganic composite materials, which are successfully used as efficient catalysts. These catalysts can display activity in aqueous, organic and green media, such as supercritical СО2 and ionic liquids [7–11]. Furthermore, a series of specific composite sorbents are also known, for example, magnetic macroporous and gel composites Dow 3N, XFS4195, C-100, C-145, Diphonix, IRC718, MN-200, and MN-202 that can effectively purify water and air from heavy metal ions [12–16].
Herein, we report on a simple method for producing a composite based on hypercrosslinked polystyrene and zinc oxide and its application as a versatile sorbent for efficient sorption of hydrogen sulfide, cadaverine, putrescine, indole, skatole, benzene, and methanol from the air at room temperature.
Results and discussion
The zinc-containing composite was obtained by filling of the pores of MN 202 industrial hypercrosslinked polystyrene sorbent with an aqueous solution of zinc chloride followed by conversion of zinc chloride to zinc hydroxide inside the polymer matrix. The subsequent drying of the composite at 120 °С led to the formation of zinc oxide from its hydroxide, which was confirmed by X-ray powder diffraction (λ[CuKα] = 1.5406 Å). Thus, the X-ray pattern shows diffraction peaks at 2θ = 31.70, 33.75, 36.23, 47.52, 56.55, 62.80, 67.89, and 69.05°, which are characteristic of zinc oxide (zincite, Fig. 1) [17, 18]. The particle sizes of this phase, calculated from the integral reflex intensities using Lvol-IB approximation, indicated the formation of zinc oxide with the mean size of coherent scattering region of 24.4(8) nm. Besides zincite, the sample contains also an admixture of another crystalline phase, which we failed to identify. According to the results of X-ray fluorescence analysis, the content of zinc in the sample was 2.9%.
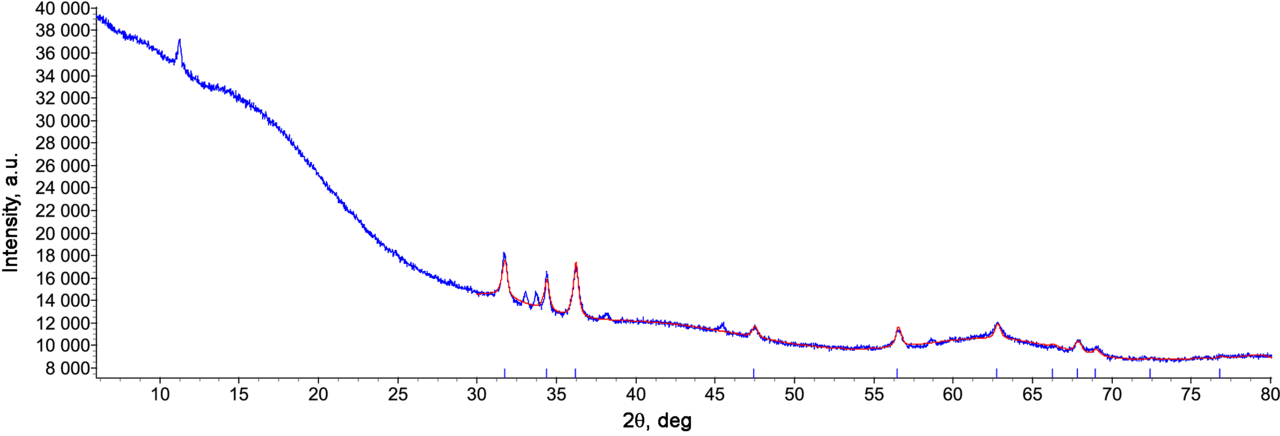
Figure 1. X-ray diffraction pattern of the resulting composite. A blue line corresponds to the experimental curve;
a red line corresponds to the curve calculated for zincite.
The composite bearing zinc oxide was studied as a hydrogen sulfide absorber. It should be noted that hydrogen sulfide is a naturally occurring component of most of the natural gas [19] and oil [20] fields. It is also present in the products of degradation of natural remains. Hydrogen sulfide has an extremely foul odor; it is toxic and corrosive for metal constructions including gas pipe lines, which requires the development of technologies for its removal from the mentioned media [19, 21]. Nowadays, hydrogen sulfide is removed mainly using di- or triamines [19, 22]. To assess the feasibility of absorption of H2S, an excess of this gas, obtained by the reaction of iron(II) sulfide with 30% sulfuric acid according to the standard procedure using Kipp's apparatus, was passed through a layer of the composite granules. The sample obtained after the passing of hydrogen sulfide was characterized by elemental analyses. The sulfur content was found to be 2.37 wt %, while the zinc content composed 2.3%, which, according to the calculations, indicates the formation of zinc disulfide [23].
The zinc-containing composite was tested for sorption capacity towards methanol and benzene (Fig. 2). It is noteworthy that these solvents are widely used in industry, although both of them are toxic in liquid and gaseous states and benzene, in addition, exhibits tumorigenicity. The highest sorption capacity was demonstrated towards methanol: up to
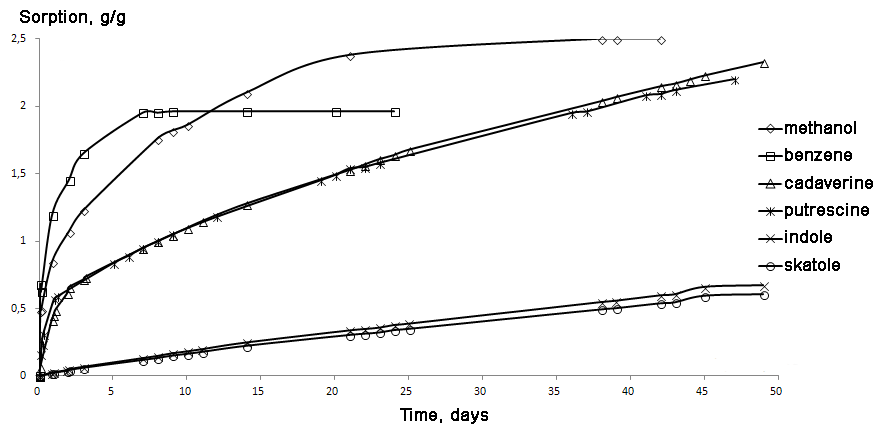
Figure 2. Sorption of organic compounds by the composite obtained.
Experimental
Preparation of the zinc-containing composite based on a Macronet MN 202 sorbent
Zinc chloride (476 mg, 3.5 mmol) was dissolved in water (20 mL). Then, a dry Purolite Macronet MN 202 sorbent (specific surface area 900 m/g2, micropores 0.6 nm, mesopores 21 nm) was added, and the resulting mixture was left under ambient conditions for 10 min. After addition of a solution of KOH (392 mg, 7 mmol) in water (20 mL), the sorbent was rinsed with water on a glass filter (3 × 20 mL). The composite was dried on the filter and then in a drying oven at 120 °С for 1 h. The samples were analyzed using a Bruker D8 Advanced diffractometer (λ[CuKa] = 1.5406 Å) and Innov-X α-2000 NMR spectrometer.
Assessment of the sorption and kinetic characteristics of the zinc-containing composite relative to sorption of organic solvents and amines
A sample of the corresponding sorbent (1.5 g) was placed in a weighing bottle and preliminarily dried at 110 °С for 1 h. Then, the bottle was placed in a desiccator and cooled. A 1 g sample was taken with an accuracy of up to 0.001 g and placed in a thin-wall porous basket with the volume of 4 mL and the known mass and hanged over benzene, methanol, cadaverine, putrescine, indole, and skatole (7 g) in a 100 mL flat-bottom flask. The mass change was defined during a certain period of time (see Fig. 2).
Conclusions
Hence, a simple technique for introducing zinc oxide into the matrix of large-scale industrial hypercrosslinked sorbent was developed. The resulting composite can facilitate a solution of a broad spectrum of problems associated with purification of air from hydrogen sulfide, toxic organic solvents, and foul-smelling products of degradation of natural remains: putrescine, cadaverine, indole, and skatole. Taking into account the above-mentioned and the well-known antimicrobial activity of zinc oxide towards gram-positive and gram-negative bacteria, the sorbent holds great promise for the application in air purification filters, conditioners, medical protecting items used in domestic and public places, transport, food production zones, stores, for personal protective devices, and at the centers for processing of household wastes [24, 25].
Acknowledgements
This work was supported by the Russian Foundation for Basic Research, project no. 18-29-25007.
References
- H. Kan, R. Chen, S. Tong, Environ. Int., 2012, 42, 10–19. DOI: 10.1016/j.envint.2011.03.003
- H. Akimoto, Science, 2003, 302, 1716–1719. DOI: 10.1126/science.1092666
- C. Pénard-Morand, I. Annesi-Maesano, Breathe, 2004, 1, 108–119. DOI:
- R. T. Yang, Adsorbents: Fundamentals and Applications, Wiley, Hoboken, 2003.
- V. Davankov, M. Tsyurupa, Hypercrosslinked Polymeric Networks and Adsorbing Materials, Elsevier, Oxford, 2011.
- L. Tan, B. Tan, Chem. Soc. Rev., 2017, 46, 3322–3356. DOI: 10.1039/C6CS00851H
- S. N. Sidorov, I. V. Volkov, V. A. Davankov, M. P. Tsyurupa, P. M. Valetsky, L. M. Bronstein, R. Karlinsey, J. W. Zwanziger, V. G. Matveeva, E. M. Sulman, N. V. Lakina, E. A. Wilder, R. J. Spontak, J. Am. Chem. Soc., 2001, 123, 10502–10510. DOI: 10.1021/ja0107834
- E. Sulman, V. Doluda, S. Dzwigaj, E. Marceau, L. Kustov, O. Tkachenko, A. Bykov, V. Matveeva, M. Sulman, N. Lakina, J. Mol. Catal. A: Chem., 2007, 278, 112–119. DOI: 10.1016/j.molcata.2007.08.029
- V. N. Sapunov, A. A. Stepacheva, E. M. Sulman, J. Wärnå, P. Mäki-Arvela, M. G. Sulman, A. I. Sidorov, B. D. Stein, D. Yu. Murzin, V. G. Matveeva, J. Ind. Eng. Chem., 2017, 46, 426–435. DOI: 10.1016/j.jiec.2016.11.013
- S. E. Lyubimov, A. A. Vasil'ev, A. A. Korlyukov, M. M. Ilyin, S. A. Pisarev, V. V. Matveev, A. E. Chalykh, S. G. Zlotin, V. A. Davankov, React. Funct. Polym., 2009, 69, 755–758. DOI: 10.1016/j.reactfunctpolym.2009.06.004
- S. E. Lyubimov, E. A. Rastorguev, K. I. Lubentsova, A. A. Korlyukov, V. A. Davankov, Tetrahedron Lett., 2013, 54, 1116–1119. DOI: 10.1016/j.tetlet.2012.12.063
- D. Leun, A. K. SenGupta, Environ. Sci. Technol., 2000, 34, 3276–3282. DOI: 10.1021/es0010148
- S. E. Lyubimov, L. A. Pavlova, M. V. Sokolovskaya, A. A. Korlyukov, V. A. Davankov, Russ. Chem. Bull., 2019, 68, 1599–1602. DOI: 10.1007/s11172-019-2598-9
- S. E. Lyubimov, M. V. Sokolovskaya, P. V. Zhemchugov, L. A. Pavlova, S. P. Kutumov, V. A. Davankov, Russ. Chem. Bull., 2020, 69, 712–714. DOI: 10.1007/s11172-020-2822-7
- J. Wu, L. Zhang, C. Long, Q. Zhang, J. Chem. Eng. Data, 2012, 57, 3426–3433. DOI: 10.1021/je300550x
- L. Jia, J. Ma, Q. Shi, C. Long, Environ. Sci. Technol., 2017, 51, 522–530. DOI: 10.1021/acs.est.6b05039
- N. Uekawa, R. Yamashita, Y. J. Wu, K. Kakegawa, Phys. Chem. Chem. Phys., 2004, 6, 442–446. DOI: 10.1039/B310306D
- S. M. Pourmortazavi, Z. Marashianpour, M. S. Karimi, M. Mohammad-Zadeh, J. Mol. Struct., 2015, 1099, 232–238. DOI: 10.1016/j.molstruc.2015.06.044
- L. Wang, R. T. Yang, Front. Chem. Sci. Eng., 2014, 8, 8–19. DOI: 10.1007/s11705-014-1411-4
- R. Javadli, A. de Klerk, Appl. Petrochem. Res., 2012, 1, 3–19. DOI: 10.1007/s13203-012-0006-6
- J. Jiang, A. Chan, S. Ali, A. Saha, K. J. Haushalter, W.-L. M. Lam, M. Glasheen, J. Parker, M. Brenner, S. B. Mahon, H. H. Patel, R. Ambasudhan, S. A. Lipton, R. B. Pilz, G. R. Boss, Sci. Rep., 2016, 6, 20831. DOI: 10.1038/srep20831
- R. Abdulrahman, I. Kamal, J. Ali, Int. J. Eng. Trends Technol., 2015, 28, 214–218. DOI: 10.14445/22315381/IJETT-V28P242
- T. A. Bither, R. J. Bouchard, W. H. Cloud, P. C. Donohue, W. J. Siemons, Inorg Chem., 1968, 7, 2208–2220. DOI: 10.1021/ic50069a008
- N. Jones, B. Ray, K. T. Ranjit, A. C. Manna, FEMS Microbiol. Lett., 2008, 279, 71–76. DOI: 10.1111/j.1574-6968.2007.01012.x
- R. Tankhiwale, S. K. Bajpai, Colloids Surf., B, 2012, 90, 16–20. DOI: 10.1016/j.colsurfb.2011.09.031





