2020 Volume 3 Issue 1
|
|
INEOS OPEN, 2020, 3 (1), 29–34 Journal of Nesmeyanov Institute of Organoelement Compounds DOI: 10.32931/io2004a |
|
Rheological and Rheokinetic Properties of Compositions Based on a Butyl Rubber,
an MQ Copolymer, and Polymethylsilsesquioxane
a Topchiev Institute of Petrochemical Synthesis, Russian Academy of Sciences, Leninskii pr. 29, Moscow, 119991 Russia
b Enikolopov Institute of Synthetic Polymeric Materials, Russian Academy of Sciences, ul. Profsoyuznaya 70, Moscow, 117393 Russia
c Mendeleev University of Chemical Technology of Russia, Miusskaya pl. 9, Moscow, 125047 Russia
Corresponding author: M. V. Mironova, e-mail: mvmironova@ips.ac.ru
Received 23 February 2020; accepted 30 April 2020
Abstract
In order to develop the compositions based on a new generation of environmentally friendly fillers that can improve mechanical characteristics, rubber compounds based on a model butyl rubber, polymethylsilsesquioxane (PMSS), and an MQ copolymer are explored. The effect of the organosilicon fillers on the rheological properties of the resulting rubber-based suspensions is evaluated. It is found that the introduction of 20 wt % of PMSS into the rubber already leads to the viscosity anomaly, while the MQ copolymer does not produce such an effect. The variation range of the moduli during a cross-linking process is significantly higher for the compositions containing the MQ resin. At the same time, absolute magnitudes of the storage and loss moduli for the systems with PMSS are much higher. One of the reasons for this behavior may be stronger adsorption of the rubber molecules on PMSS particles, which leads to the formation of a mixed network of the rubber with physical and chemical bonds.
Key words: organosilicon polymers, viscoelastic properties, rheokinetics.
Introduction
Traditionally, rubber compounds consist of elastomers, which are capable of cross-linking under the action of certain agents, and microparticles of carbon black, which improve the mechanical characteristics of the resulting rubber [1]. The last two decades of the 20th century witnessed the rapid development of approaches to partial or complete replacement of carbon black for various grades of silicon dioxide or fumed silica [2–7]. The tires based on these rubbers provided substantial environmental benefits (the production of carbon black from raw hydrocarbons and the compounding of a rubber with carbon black are quite harmful, as well as the black tire marks on roads that arise during braking) and were called "green". Some technical advantages and disadvantages of the new fillers were revealed immediately. The advantages comprise fuel economy owing to the reduced rolling friction losses and the increased stability of these tires on wet roads [8, 9]. The drawbacks include the increased propensity of silica for the interaction of particles with each other and the presence of polar silanol groups on the surface, which reduce the efficiency of particle interaction with nonpolar rubbers [2].
Thus, the term "green tires" has been known at least for 25–30 years and is used mainly for the tires based on conventional organic rubbers in which silica particles are utilized as reinforcing fillers [10–13]. The first developments in this area were made by the French company Michelin: in 1996 it launched the production of tires in which most of the technical additives were replaced for silicon dioxide [14, 15]. Degussa proposed to treat the surface of silicon dioxide with bifunctional organosilicon compounds, for example, bis[3-(triethoxysilyl)propyl]tetrasulfane which can react with the silanol groups on the silica surface at high temperatures, leading to its hydrophobization and, consequently, increasing its compatibility with nonpolar rubbers and reducing the viscosity of the rubber compounds.
The first substitute for carbon black was almost environmentally friendly silicon dioxide. However, it does not provide the same strength as carbon black and, therefore, cannot completely replace the latter [16–18]. As it was already mentioned, a reason is the presence of polar moieties in silica, in particular, the silanol groups, which reduce its compatibility with nonpolar rubbers [19, 20]. In this regard, polysilsesquioxanes with organic peripheries, MQ copolymers, and other organosilicon products may be more suitable fillers for rubbers [21–24]. Retaining the reinforcing silica core, these compounds exhibit rather high compatibility with carbochain and silicone rubbers [25–27]. The application potential of the latter is not obvious yet, but they can help to achieve the high adhesion between a filler and a polymer matrix in the rubber and, thereby, to turn to completely green tires.
One of the main challenges associated with the use of the cross-linked organosilicon fillers is the assessment of their effect on the rheological properties of the rubber-based suspensions. Another problem is the analysis of the behavior of a filled system during cross-linking, both in terms of the process kinetics and the density of the resulting network junctions. The main goals of this work are to address these issues by the example of carbochain butyl rubber as a matrix and polymethylsilsesquioxane and MQ copolymer as fillers.
Results and discussion
The introduction of the filler into the polymer matrix by mixing from solution provides more uniform distribution of the additive compared to that achieved by mechanical mixing. However, at such a high filler content (20 and 40 wt %), it is difficult to identify the morphological details. Figure 1 shows as an example a micrograph of the PMSS composition. The white areas correspond to the filler phase with the particle sizes ranging from several to 50–100 microns.

Figure 1. Micrograph of Kalene 1300 filled with 40 wt % of PMSS. The scale bar is equal to 200 microns.
A butyl rubber exhibits Newtonian behavior, i.e., its viscosity is constant over a wide range of the applied shear stresses (10–104 Pa) (Fig. 2). At the stresses above 104 Pa, a decrease in the viscosity is observed, which is characteristic of flexible-chain polymers. This is due to the "spurt" effect, i.e., a transition of the surface layers to the induced rubber-like state and their sliding relative to the walls of the working unit [28, 29].

Figure 2. Dependence of the viscosity on the shear stress for the matrix polymer (1)
and the compositions containing 20 (2), 30 (3), and 40 (4) wt % of the MQ copolymer.
For the systems containing 20, 30, and 40 wt % of the MQ copolymer, the curves of deformation development upon application of the shear stress feature a traditional character. The deformation increases linearly in time, the slope of the straight lines can serve as a measure of the shear rate and, given the applied stress, also as a measure of the viscosity. The shear viscosity is expected to increase with an increase in the filler concentration (within an order of magnitude). The weak dependence of the viscosity on the stress appears and becomes more noticeable at low stresses. At the same time, the effect of a sharp decrease in the viscosity in the region of high stresses disappears. This is a fairly well-known phenomenon of a reduction in the flexibility of macromolecules as a result of interaction with the filler surface [28].
A different picture is observed for the systems with PMSS. An anomaly of the viscosity is manifested for the systems containing already 20 wt % of the filler. At 30% filling, the viscosity reaches very high values—about 108 Pa·s, and the system becomes strongly non-Newtonian (Fig. 3). In absolute terms, the viscosity of the compositions containing the PMSS filler is significantly higher than that of the compositions with the MQ copolymer.

Figure 3. Dependence of the viscosity on the shear stress for the polymer matrix (1) and the compositions containing 20 (2) and 30 (3) wt % of PMSS.
Additional information on the structure formation in the compositions with different fillers can be provided by the data of dynamic measurements.
The polymer matrix is a viscoelastic material. At room temperature in the low-frequency region, the viscous behavior prevails (the loss modulus exceeds the elastic modulus). However, at the frequencies above 200 s–1, a "crossover point" is observed, above which the elastic component exceeds the viscous one. The introduction of the MQ resin into the polymer matrix leads only to a moderate increase in the absolute values of the moduli, without changing the general character of the frequency dependences (Fig. 4).

Figure 4. Frequency dependences of the dynamic moduli for the butyl rubber (1) and its composition containing 40 wt % of the MQ copolymer (2).
Closed and open symbols refer to G' and G'', respectively.
As for the evolution of the frequency dependences of the moduli with an increase in the content of the MQ copolymer, the most noticeable growth of the moduli is observed for 20% concentration of the resin, while a further increase in the filler amount no longer causes significant changes in their values (Fig. 5). Judging by the values of the exponents of the frequency dependences of the moduli G'~w1.3 and G''~w0.9, some decrease in the exponent magnitudes relative to the classical values of 2 and 1 can be explained by a broad molar mass distribution of Kalene.


Figure 5. Frequency dependences of the dynamic moduli for the composition based on Kalene (1)
and the compositions containing 20 (2), 30 (3), and 40 (4) wt % of the MQ copolymer.
Another situation develops for the compositions with PMSS. Thus, the addition of 20 wt % of the filler significantly affects not only the values but also the character of variation of the dynamic moduli depending on the frequency (Fig. 6). For the system bearing 20 wt % of PMSS, the loss modulus exceeds the elastic one at low frequencies, i.e., a viscous reaction prevails, but, at the same time, a "crossover" is observed even at the frequency of only 5 s–1. A system with 30 wt % of PMSS already exhibits elastic properties in the whole frequency range, i.e., the storage modulus G' exceeds the loss modulus G". For the composition with the filler content of 40 wt %, the components of the complex dynamic modulus remain virtually unchanged in the entire frequency range. The value of the storage modulus reaches 2×107 Pa.

Figure 6. Frequency dependences of the dynamic moduli for the composition based on Kalene (1) and the compositions containing
20 (2), 30 (3), and 40 wt % (4) of PMSS. Closed and open symbols refer to G' and G", respectively.
The data obtained suggest that the most suitable filler for the butyl rubber from the viewpoint of rheological characteristics is the MQ copolymer. However, a key point is the behavior of all the compositions under consideration during cross-linking, i.e., when a polymer matrix converts to a rubber.
During cross-linking of the compositions based on Kalene 1300 and different fillers, the kinetics of changes in the elastic modulus and the mechanical loss tangent were analyzed. The mechanical loss tangent, tgd = G"/G', reflects the fraction of a dissipative component of the complex elastic modulus [30]. Figure 7 depicts these relationships for the compositions bearing the MQ copolymer as a filler. The use of a p-quinone dioxime derivative as a curing agent allows for the rapid cross-linking. The moduli reach constant values (a plateau) within 20–40 min. This characteristic time decreases with an increase in the filler content in the system. It should be noted that the area corresponding to the induction period does not exceed 2 (for the filled systems) or 8 (for the polymer matrix) min, which is explained by the participation of the filler in the activity of the cross-linking agent in use at the given temperature (Т = 120 °С). The values of tgd above one indicate the presence of a high dissipative component in the original system. When the cross-linking process completes, the tangent value becomes significantly less than one, which means the actual absence of flow and a predominantly elastic reaction of the cross-linked system.
 |
 |
| a | b |
Figure 7. Changes in the elastic modulus (a) and the loss tangent (b) during curing of Kalene (1) and the compositions containing
20 (2), 30 (3), and 40 (4) wt % of the MQ copolymer. Temperature is 120 °C.
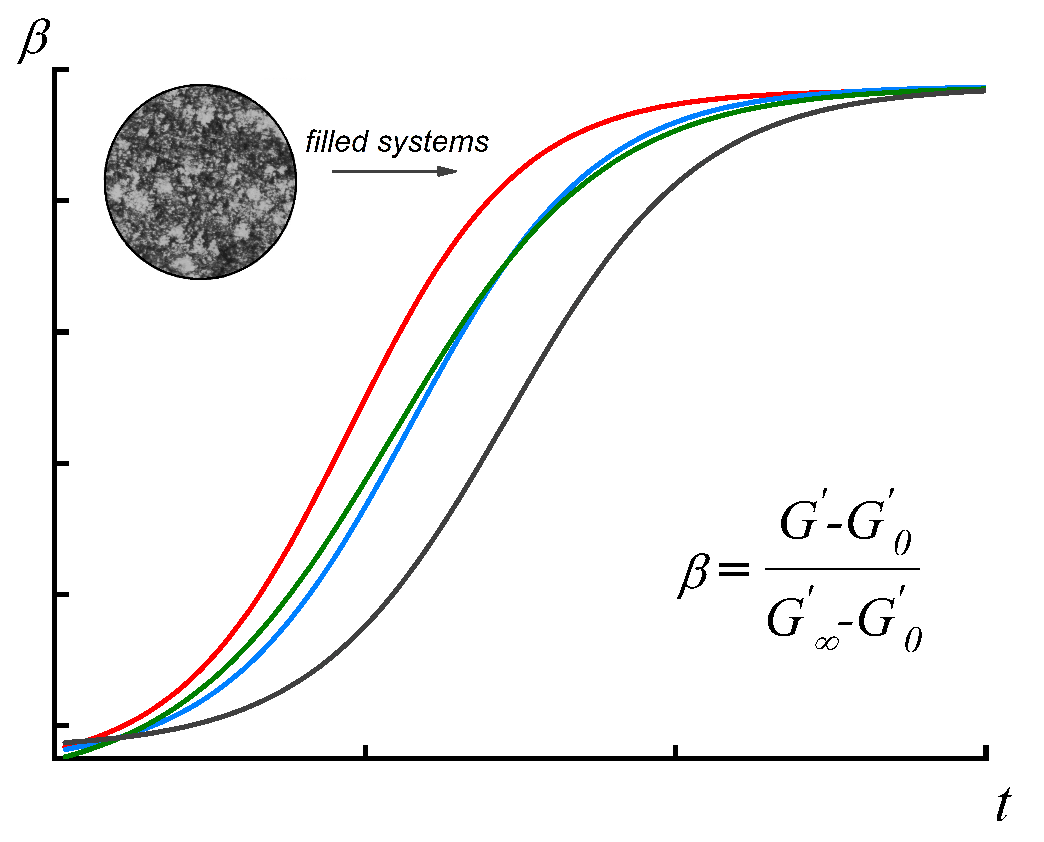
From the dependences of the elastic modulus on the curing time, the characteristic values reflecting the depth of the process can be found. For this purpose, the rheological degree of conversion β is usually used [31]. It is expressed as follows:

where

Figure 8. Dependence of the rheological degree of conversion β on the cross-linking duration for Kalene (1)
and the compositions containing 20 (2), 30 (3), and 40 (4) wt % of the MQ copolymer.
The nature of changes in the elastic modulus and the mechanical loss tangent during curing of the compositions filled with PMSS is presented in Fig. 9. Despite the fact that the cross-linking rates for all the systems remain high, the extent of changes in the modulus for this filler becomes significantly lower than that for the compositions bearing the MQ copolymer. In addition, a magnitude of the modulus at the plateau is considerably higher than that observed in the previous case, approaching 106 Pa. The value of the tangent not only during the process but also at its beginning remains less than one and significantly decreases on passing from 20 to 30% filling. The area corresponding to the induction period for these compositions could not be fixed at all due to the high rate of the process.
 |
 |
| a | b |
Figure 9. Changes in the elastic modulus (a) and the mechanical loss tangent (b) during curing of the compositions filled with 20 (1) and 30 (2) wt % PMSS.
Figure 10 shows the dependence of the rheological degree of conversion on the curing time for the compositions with PMSS. As in the case of the compositions based on the MQ resins, the β(t) curves shift to the region of shorter times as the filling degree increases.

Figure 10. Dependence of the rheological degree of conversion β on the curing duration for Kalene 1300 (1)
and the compositions filled with 20 (2) or 30 (3) wt % of PMSS.
The following considerations can be used to explain the results obtained. When the fillers are introduced into the butyl rubber, the polymer macromolecules are separated into free ones and bound into adsorption layers around the particles. The ratio of these changes depends on the intensity of adsorption. Judging by the rheological data, the adsorption proceeds most intensively in the case of PMSS. During curing, free macromolecules are most active in interacting with the cross-linking agent, the proportion of which is the highest one for the compositions with the MQ resins. For this reason, the extent of changes in the elastic modulus and the mechanical loss tangent for these compositions is significantly higher than that for the other systems. The composition with PMSS is the least active one in terms of chemical cross-linking, presumably, since the large portions of the butyl rubber macromolecules are involved in physical cross-linking due to adsorption on the organosilicon particles.
Experimental
Materials
To create the model compositions, Kalene 1300 butyl rubber (3.5% content of unsaturated isoprene units) was chosen as the polymer matrix. This copolymer is characterized by the low molar mass (4.2 × 104) and the relatively low viscosity (1300 Pa·s at T = 60 °C), which facilitates the introduction of a filler into it and allows for the room temperature rheological studies.
The following organosilicon compounds were used as the active fillers: MQ copolymers and hyperbranched polymethylsilsesquioxane. The MQ copolymers (MQ resins) have specific macromolecular structures. The compositions contain inorganic, SiO4/2, and organic, R3SiO1/2, components, which allows us to classify these compounds as hybrid nanoscale organo-inorganic materials [32]. This work was concerned with the MQ copolymer featuring 1:2 ratio of the M and Q units. It was obtained by the heterofunctional polycondensation of tetraethoxysilane and hexamethyldisiloxane in an active medium in the presence of acetyl chloride according to the scheme:
The hydroxy groups of the copolymer explored were blocked by trimethylchlorosilane, resulting in the following repeating unit:
Polymethylsilsesquioxane (PMSS) of general formula [MeSiO1.5]∞ used in this research was obtained by mixing methyltriethoxysilane (178.3 g, 1 mol) and 96% ethanol (142 g). Then, a solution of sodium hydroxide (9.1 g, 0.23 mol) in water (284 g) was added. The resulting solution was stirred for 30 min on a magnetic stirrer and left at room temperature for 15 h. The resulting gel was washed on a filter until the washings gave a neutral reaction (with phenolphthalein). This afforded a gel (700 g) with 6% of the dry residue. The gel was then diluted with acetone to 2% solution and dried in a spray dryer to give a white fine powder with the specific surface area of 520 m2/g [21]. The polymer is insoluble in common organic solvents and thermally stable up to 300 °C. These properties of the polymer are explained by a dense 3D network structure [33–35].
The selected excipients are hydrophobic and, therefore, should be well combined with the carbochain polymer matrix.
For the cross-linking of Kalene 1300, the following quinol ether was used (TU (Specifications) 6-09-11-2151-94, ANGARA-REAKTIV): 0,0-bis(1,3,5,-tri-tert-butyl-4-oxo-2,5)cyclohexadienyl-p-benzoquinone dioxime. The derivatives of p-quinone dioxime are capable of combining with the polymer and are effective low-temperature cross-linking agents for the compositions based on unsaturated rubbers [1]. A powder of the quinol ether was introduced into the composition in the amount of 5 wt %.
Methods
The active filler was added to the rubber in the amount of 20, 30, or 40 wt %. For this purpose, two methods of mechanical mixing were used: from solution and in melt. The MQ resins were introduced into the polymer matrix by mixing from solution using a vacuum rotary evaporator. The rubber was preliminarily dissolved in methyl tert-butyl ether in a PSB-Hals ultrasonic bath (operating frequency 60 kHz). Then, a solution of the MQ copolymer in the same solvent was added. The resulting mixture was evacuated at 60 °C for 2 h on a Rotavapor rotary evaporator (Buchi, Switzerland). The solution mixing ensures uniform distribution of the components in the polymer matrix. The uniform distribution of PMSS in the rubber was achieved by the prolonged mechanical mixing with a three-roll laboratory mixer (EXAKT, Otto Herrmann, Germany).
The morphology of the filled systems was studied using a Biomed 6PO laboratory optical microscope (Biomed Service, Russia). The rheological properties of the filled compositions were measured using a Physica MCR 301 rotational rheometer (Anton Paar, Austria). In these experiments, a plate–plate operating unit with the diameter of a rotating plate of 25 mm was used. The flow curves of the compositions were obtained in two ways: by a stepwise variation of the controlled shear stress in the range from 100 to 40000 Pa (the duration of the experiment for each point was 1 min) and by the long-term measurements at the given constant shear stress in the same range. A measure of the viscosity was the shear rate when it reached a constant value, i.e., after the dependence of the strain on time at a constant slope. The dynamic tests of the samples were carried out in the frequency range of 6×10–1–6×102 s–1 with the constant oscillation amplitude of 1%, which ensured the strain of the samples within the region of linear viscoelasticity. All the tests were carried out at 25 °C.
The kinetics of cross-linking was studied by measuring the dynamic moduli G' and G" at 120 °C on a RheoStress 600 rotational rheometer (Thermo Haake, Germany) in time. The strain amplitude was 1%; the frequency was 1 Hz.
Conclusions
The rheological and rheokinetic studies of the compositions based on the model butyl rubber and various organosilicon fillers revealed the main structural features of the highly filled systems containing 20–40% of particles prepared by different methods. From the viewpoint of viscoelastic properties, the compositions with PMSS are elasto-viscous, since the elastic modulus starting from the concentration of 30% exceeds the loss modulus. This feature was also observed in rheokinetic experiments that describe the evolution of the dynamic moduli during cross-linking of the butyl rubber with the p-quinone dioxime derivative. The lack of an ascending branch of the dependence of the elastic modulus on time suggested the presence of a dense network of physical connections in the systems containing PMSS. Thus, the new green fillers demonstrated great potential for tuning the properties of the model rubber compositions. Although the practical application of the compositions obtained has not been demonstrated yet, the investigation performed opened the way to a new huge area of combinatorial tuning of the explored rubber compositions based on new green fillers.
Acknowledgements
This work was supported by the Russian Foundation for Basic Research, project no. 18-33-20247.
The synthesis of the fillers was supported by the Russian Foundation for Basic Research, project no 18-29-25053.
References
- Science and Technology of Rubber, 3rd ed., J. E. Mark, B. Erman, F. R. Eirich (Eds.), Acad. Press, Amsterdam, Elsevier, 2005.
- K. L. Kandyrin, Kauch. Rezina, 2011, 4, 37–42.
- EP Patent 0705722 A1, 1994.
- US Patent 6020402 A, 2000.
- EP Patent 0830954 B1, 1996.
- US Patent 5719207 A, 1998.
- US Patent 6111008 A, 2000.
- EP Patent 0881252 A1, 1997.
- US Patent 20150144242 A1, 2015.
- US Patent 6378582, 2002.
- US Patent 7250463 B2, 2007.
- US Patent 20080319125 A1, 2008.
- FR Patent 3047735 A1, 2017.
- FR Patent 2740778 A1, 1997.
- FR Patent 2744127 A1, 1996.
- Yu. A. Gamlitsky, Nanoscie. Technol. Int. J., 2013, 4, 179–196 DOI: 10.1615/NanomechanicsSciTechnolIntJ.v4.i3.10
- L. V. Sokolova, A. V. Losev, Kauch. Rezina, 2019, 78, 220–227.
- W. Kaewsakul, K. Sahakaro, W. K. Dierkes, J. W. M. Noordermeer, Polym. Eng. Sci., 2015, 55, 836–842. DOI: 10.1002/pen.23949
- L. Chen, Z. Jia, Y. Tang, L. Wu, Y. Luo, D. Jia, Compos. Sci. Technol., 2017, 144, 11–17. DOI: 10.1016/j.compscitech.2016.11.005
- M. Castellano, L. Conzatti, A. Turturro, G. Costa, G. Busca, J. Phys. Chem. B., 2007, 111, 4495–4502. DOI: 10.1021/jp0702144
- I. B. Meshkov, N. G. Mazhorova, P. V. Zhemchugov, A. A. Kalinina, S. G. Vasil'ev, A. V. Bystrova, S. E. Lyubimov, A. S. Tereshchenko, A. M. Muzafarov, INEOS OPEN, 2019, 2, 140–144. DOI: 10.32931/io1920a
- O. A. Serenko, V. G. Shevchenko, A. S. Zhiltsov, V. E. Chuprakov, T. V. Zaderenko, O. T. Gritsenko, M. V. Mironova, O. B. Gorbatsevich, V. V. Kazakova, V. G. Kulichikhin, A. M. Muzafarov, Nanotechnol. Russ., 2013, 8, 81–91. DOI: 10.1134/S1995078013010138
- O. A. Serenko, M. V. Mironova, N. A. Novozhilova, P. V. Strashnov, E. V. Getmanova, A. A. Askadskii, V. G. Shevchenko, V. G. Kulichikhin, A. M. Muzafarov, Mater. Chem. Phys., 2015, 156, 16–28. DOI: 10.1016/j.matchemphys.2015.02.013
- J. Ji, X. Ge, X. Pang, R. Liu, S. Wen, J. Sun, W. Liang, J. Ge, X. Chen, Polymers, 2019, 11, 1142. DOI: 10.3390/polym11071142
- E. A. Karpukhina, S. O. Ilyin, V. V. Makarova, I. B. Meshkov, V. G. Kulichikhin, Polym. Sci., Ser. A., 2014, 56, 798–811. DOI: 10.1134/S0965545X14060066
- O. A. Serenko, A. M. Muzafarov, Polym. Sci., Ser. C, 2016, 58, 93–101. DOI: 10.1134/S1811238216010112
- F. Sun, Y. Hu, H.-G. Du, J. Appl. Polym. Sci., 2012, 125, 3532–3536. DOI: 10.1002/app.35194
- G. V. Vinogradov, A. Ya. Malkin, Rheology of Polymers, Khimiya, Moscow, 1977 [in Russian].
- A. Ya. Malkin, A. I. Isaev, Rheology: Concepts, Methods, and Applications, ChemTec Publ., Toronto, 2017.
- V. E. Dreval, S. V. Emelyanov, V. A. Shershnev, V. G. Kulichikhin, A. E. Chalykh, A. D. Aliev, M. V. Vocal, Vysokomol. Soedin., Ser. A, 2005, 47, 730–736.
- A. Ya. Malkin, S. G. Kulichikhin, Rheology in the Processes of Formation and Transformation of Polymers, Khimiya, Moscow, 1985 [in Russian].
- E. Tatarinova, N. Vasilenko, A. Muzafarov, Molecules, 2017, 22, 1768. DOI: 10.3390/molecules22101768
- V. V. Kazakova, V. D. Myakushev, T. V. Strelkova, A. M. Muzafarov, Vysokomol. Soedin., Ser. A, 1999, 41, 283–289.
- D. Migulin, E. Tatarinova, I. Meshkov, G. Cherkaev, N. Vasilenko, M. Buzin, A. Muzafarov, Polym Int., 2016, 65, 72–83. DOI: 10.1002/pi.5029
- A. I. Amirova, O. V. Golub, D. A. Migulin, A. M. Muzafarov, Int. J. Polym. Anal. Charact., 2016, 21, 214–220. DOI: 10.1080/1023666X.2016.1136867





