2019 Volume 2 Issue 5
|
|
INEOS OPEN, 2019, 2 (5), 167–171 Journal of Nesmeyanov Institute of Organoelement Compounds DOI: 10.32931/io1924a |
|
Polynaphthoylenebenzimidazoles and Their Metal Derivatives
V. G. Krasovskii,b M. Yu. Yablokov,c and V. S. Papkova
a Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, Moscow, 119991 Russia
b Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Leninskii pr. 47, Moscow, 119991 Russia
c Enikolopov Institute of Synthetic Polymeric Materials, Russian Academy of Sciences, ul. Profsoyuznaya 70, Moscow, 117393 Russia
Corresponding author: E. G. Bulycheva, e-mail: bulychev@ineos.ac.ru
Received 20 December 2019; accepted 27 January 2020
Abstract
A series of polynaphthoylenebenzimidazoles (PNBIs) are synthesized by high-temperature catalytic polyheterocyclization. Their polymer-analogous transformations lead to sulfonated derivatives and salts bearing K, Ca, and Zn ions. The resulting polymers are characterized by elemental and thermogravimetric analyses and IR spectroscopy. Electron beam dispersion of the metal derivatives of PNBI affords coatings. The results of the preliminary evaluation of their electrical properties are presented.
Key words: sulfonation, polynaphthoylenebenzimidazoles, ionomers, films, electron beam dispersion.
Introduction
Modern technology requires polymeric materials that possess improved heat and thermal stability, high resistance to chemical reagents and stress-strain properties and can be processed by the known technological approaches. Further progress in this field is associated with the creation of aromatic high-molecular heterocyclic compounds, such as polynaphthoylenebenzimidazoles. Owing to a combination of the mentioned characteristics, these polymers may find application as thermally stable film materials, coatings, high-temperature proton conducting membranes, and so on [1–3]. Unfortunately, the low solubility of PNBIs in common organic solvents hampers their processing in solution, while the high glass-transition temperatures encumber their processing in melts.
Earlier it was shown that the desired properties of PNBIs can be imparted by the introduction of bridging units [4]. Furthermore, it was reported that the introduction of sulfo groups into PNBIs changes dramatically their solubility [5].
Herein, we report on the synthesis of new PNBIs and the effect of additional bridging units on the physical properties of the resulting polymers. The main focus is on the investigation of the properties of sulfonated PNBIs and their salts. An original approach to the production of coatings from the metal derivatives of PNBIs by electron beam dispersion is presented.
Results and discussion
The directed synthesis of a series of new PNBIs was accomplished by the high-temperature catalytic polyheterocyclization [6, 7] of the following tetraamines: 3,3',4,4'-tetraaminodiphenyloxide (I), 2-bis(3,4-diaminophenyl)-hexafluoropropane (II), 2,2-bis[4-(3,4-diaminophenoxy)-phenyl]hexafluoropropane (III), and 1,3-bis(3,4-diaminophenoxy)benzene (IV) with naphthalene-1,4,5,8-tetracarboxylic acid dianhydride (V) or the related derivative of 1,3-bis(1,8-dicarboxynaphthaloyl-4-)benzene (VI) (Scheme 1).
Scheme 1
The investigation of thermal properties of the resulting PNBIs showed that all the polymers feature high decomposition onset and glass-transition temperatures which fall within 450–550 and 350–370 °С, respectively (Table 1).
Table 1. Characteristics of the resulting polynaphthoylenebenzimidazoles
|
Polymer |
–Х– |
Yield, % |
ηred, dL/ga |
Тg, °Сb |
Тdec, °Сc |
|
|
1 |
I |
V |
89 |
0.9 |
370 |
550 |
|
2 |
III |
V |
95 |
1.2 |
363 |
502 |
|
3 |
I |
VI |
93 |
1.5 |
360 |
450 |
|
4 |
II |
VI |
97 |
1.7 |
355 |
501 |
|
5 |
III |
VI |
91 |
0.5 |
350 |
498 |
|
6 |
IV |
VI |
88 |
0.2 |
348 |
502 |
a reduced viscosity of the polymer solution at the concentration of 0.5 g/dL at 25 °С;
b glass-transition temperature defined from the thermomechanical curves;
c decomposition onset temperature (5% of the mass loss according to the TGA curves).
The polynaphthoylenebenzimidazoles based on precursors I and V are soluble only in sulfuric acid; the other PNBIs are soluble also in a tetrachloroethane–phenol mixture (3:1). Such a limited number of solvents hampers the practical application of these polymers. To improve the solubility of PNBIs, we accomplished their sulfonation via a polymer-analogous transformation upon treatment with a mixture of sulfuric acid and oleum. This afforded the sulfonatad derivatives of PNBIs (SPNBIs) that have different sulfur contents (Scheme 2, Table 2).
Scheme 2
Table 2. Properties of sulfonated polynaphthoylenebenzimidazoles
|
Polymera |
Sulfonation time, h |
|||||||
|
5 |
10 |
15 |
25 |
|||||
|
S, %b |
ηred, dL/gc |
S, %b |
ηred, dL/gc |
S, %b |
ηred, dL/gc |
S, %b |
ηred, dL/gc |
|
|
SPNBI-I,V |
2.3 |
– |
3.6 |
– |
5.4 |
0.4 |
6.5 |
0.6 |
|
SPNBI-III,V |
3.8 |
1.2 |
3.6 |
1.3 |
4.0 |
1.1 |
4.1 |
1.2 |
|
SPNBI-I,VI |
3.8 |
– |
4.5 |
– |
5.0 |
0.2 |
8.5 |
0.8 |
|
SPNBI-II,VI |
4.0 |
1.7 |
4.2 |
2.1 |
4.4 |
2.7 |
– |
– |
|
SPNBI-III,VI |
– |
– |
– |
– |
3.9 |
– |
5.5 |
0.4 |
|
SPNBI-IV,VI |
– |
– |
– |
– |
1.5 |
– |
2.2 |
– |
a initial PNBIs for the synthesis correspond to those presented in Scheme 1;
b sulfur content according to the elemental analysis data, wt %;
c reduced viscosity of N-methyl-2-pyrrolidone (NMP) solution at the concentration of 0.5 g/dL at 25 °С.
The sulfonation of PNBIs consisted of several stages: the starting PNBI was dissolved in sulfuric acid at room temperature under vigorous stirring until the formation of 7–8 wt % solution, then oleum was added in 1:3 ratio by volume, the resulting mixture was stirred for 1 h, then heated to
The sulfonation of PNBI affects also the character of thermal decomposition during investigation by TGA. Figure 1 depicts the TGA curves of PNBI-I,VI (Table 1) and SPNBI on its base, which bear different sulfur contents (Table 2). As can be seen, the TGA curves of SPNBIs contain several mass loss regions. The former, which completes in the vicinity of 200 °С, corresponds to the removal of moisture absorbed from the air.

Figure 1. TGA curves of PNBI-I,VI (1), SPNBI-I,VI-3.8%S (2), SPNBI-I,VI-4.5%S (3), SPNBI-I,VI-5.0%S (4), SPNBI-I,VI-8.5%S (5), and SPNBI-I,VI-5.0%S heated in argon at 340 °С for 1.5 h (6) (heating in air at the rate of 10 °С/min). The sulfur contents are presented in wt %.
The next step of the mass loss of SPNBI-I,VI-3.8–8.5%S is located in the temperature range of 350–450 °С. The mass loss at this stage ranges from 5 to 10 wt % and grows with an increase in the sulfur content in SPNBI. Based on the literature data [8], it can be assumed that this stage of the mass loss corresponds to desulfonation, which results in the formation of intermolecular –SO2– bridges.
This assumption was checked using IR spectroscopy and TGA. For this purpose, SPNBI-I,VI and SPNBI-II,VI were heated in argon at 300 °С for 1.5 h. Note that the stage of the mass loss, which is likely to refer to desulfonation, degenerates on the TGA curve of thermally processed SPNBI-I,VI (Fig. 1, curve 6). The main changes in the IR spectra of heated SPNBI (Figs. 2, 3) were observed at 1057 and 1354 cm–1—the regions characteristic for the stretching vibrations of SO3 and SO2 groups in sulfonic acids and sulfones, respectively.
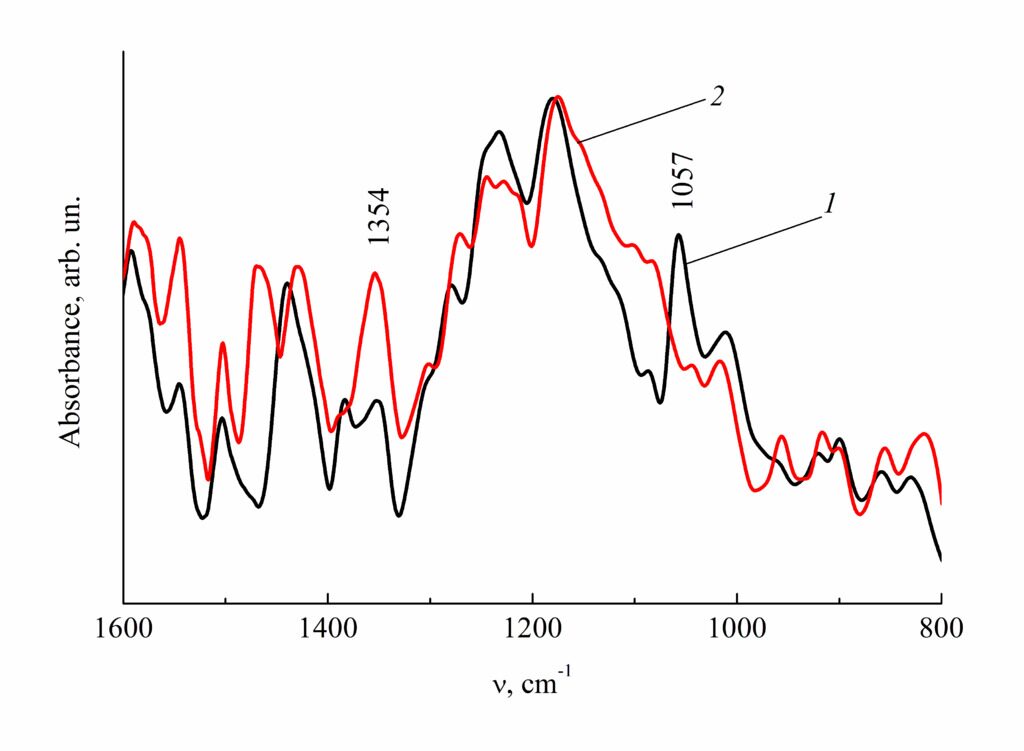
Figure 2. IR spectra of SPNBI-I,VI-5.0%S (1) and the sample obtained after heating in argon at 300 °С for 1.5 h (2).
The sulfur content is presented in wt %.
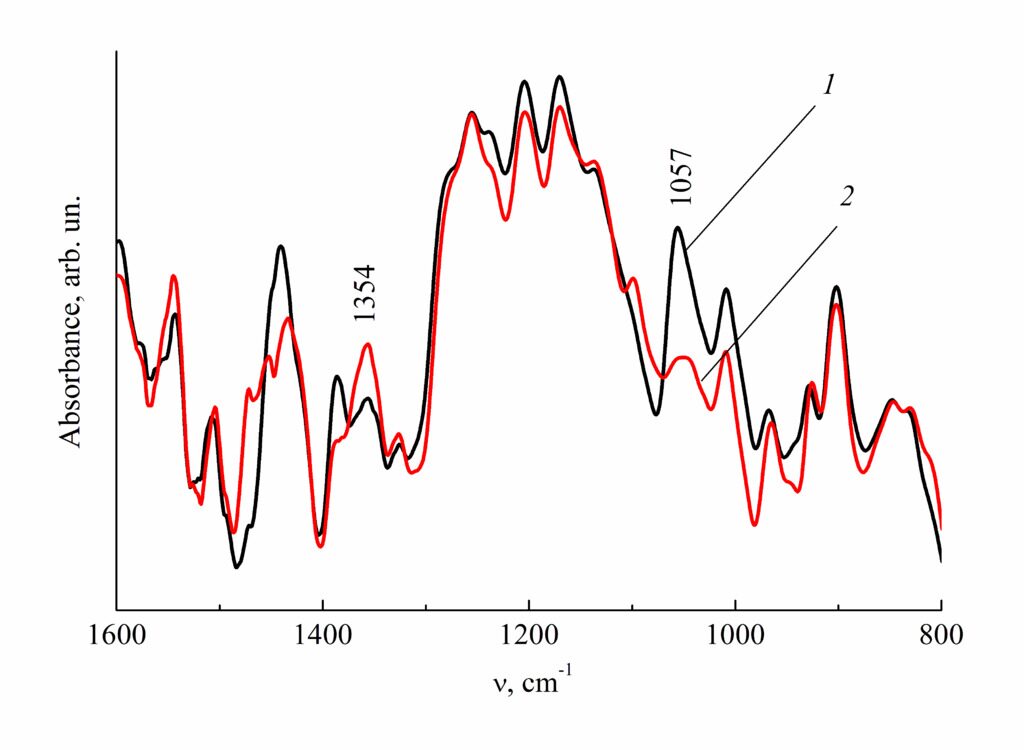
Figure 3. IR spectra of SPNBI-II,VI-4.4%S (1) and the sample obtained after heating in argon at 300 °С for 1.5 h (2).
The sulfur content is presented in wt %.
The intensity of the band at 1057 cm–1 reduces and that of the band at 1354 cm–1 increases, which can be caused by a reduction in the amount of sulfo groups and an increase in the amount of sulfone groups. The polymers heated at the mentioned temperatures do not dissolve neither in sulfuric acid nor in NMP. This indicates the formation of network structures.
The salts based on SPNBI were obtained under heterogeneous conditions according to the previously developed procedure (Scheme 3) [5].
Scheme 3
In the case of the potassium salts, the powder samples of SPNBIs were mixed with 5M aqueous solution of potassium hydroxide. The reaction was carried out for 7 days under stirring; then the resulting powder was washed with water (to neutral pH) and dried at 150 °С to the constant mass. The synthesis of the calcium salt was accomplished upon interaction of 20 wt % aqueous solution of CaCl2 with powder SPNBI for 10 days at room temperature, followed by rinsing of the resulting product with water to neutral reaction and drying at 150 °С to the constant mass. The zinc salt of SPNBI was synthesized analogously. The substitution degree of hydrogen atoms for sulfo groups was calculated from the metal content in the sample, which was defined using flame emission spectrometry (Table 3).
Table 3. Metal contents in the salt forms of SPNBIs
|
SPNBI |
Metal content, wt % |
|||||
|
K |
Ca |
Zn |
||||
|
Calcd |
Found |
Calcd |
Found |
Calcd |
Found |
|
|
SPNBI-I,VI, |
4.6 |
2.1 |
|
|
|
|
|
SPNBI-I,VI, |
5.4 |
5.0 |
|
|
|
|
|
SPNBI-I,VI, |
6.1 |
4.7 |
6.2 |
2.5 |
8.3 |
1.5 |
|
SPNBI-III,VI, |
4.2 |
1.5 |
|
|
|
|
Table 3 shows that almost complete substitution of the hydrogen atoms of sulfo groups for the metal ions was reached in the case of SPNBI-I,VI (10 h), whereas the other salts feature somewhat reduced metal contents compared to the calculated values. Such a difference can be caused by the statistical disposition of the sulfo groups along the polymer chain and, as a consequence, different availability of these groups for the interaction with the metal-containing reagent during heterogeneous reactions, which occur at the interface. It should be noted that we failed to isolate the potassium salt of SPNBI-I,VI (25 h), since it is highly soluble in an aqueous solution of KOH.
According to the results of TGA studies (Fig. 4), the SPNBI salts can readily absorb moisture from the air, which removal, as well as in the case of the sulfo derivatives of PNBIs, occurs in a wide temperature range up to 200 °С. The higher the amount of residual sulfo groups in the polymer salts, the higher the content of absorbed moisture. Presumably, the region of the mass loss within 380–480 °С on the TGA curves of the salts is connected with the residual desulfonation. Note that the onset temperature of the main decomposition stage for the salts of SPNBI-I,VI (without consideration of moisture absorbed from the air) composes 480 °С.

Figure 4. TGA curves of SPNBI-I,VI-2.1%К (1), SPNBI-I,VI-4.7%К (2), SPNBI-I,VI-5.0%К (3), SPNBI-I,VI-1.5%Zn (4), SPNBI-I,VI-2.5%Ca (5),
and SPNBI-III,VI-1.5%К (6) (heating in air at the rate of 10 °С/min). The metal contents are presented in wt %.
The DSC curves did not reveal changes connected with devitrification. Obviously, the glass-transition temperatures of SPNBIs are higher than their decomposition points.
It was found that all the resulting SPNBI salts are insoluble in organic solvents. This fact stipulated us to search for alternative routes for the production of film materials that differ from the conventional solution-based methods. To form the coatings on different substrates, we chose the deposition from an active gas phase by electron beam dispersion under vacuum, which was already recommended for the application of coatings from polytetrafluoroethylene and chitosan [9–12]. The procedure for the formation of coatings is described in detail in Ref. [9]. According to the AFM data, the thicknesses of the coatings applied on silicon plates composed 76, 21, 31, 36, and 36 nm for PNBI-I,VI, SPNBI-I,VI, SPNBI-I,VI-4.7%К, SPNBI-I,VI-2.5%Са, and SPNBI-I,VI-1.5%Zn, respectively.
The IR spectra of PNBI-I,VI and its salts (Fig. 5a) contain all the bands characteristic for PNBI and SPNBI (vide supra). Furthermore, it should be noted that these spectra show intensive bands at 1055 cm–1, which are typical for the stretching vibrations of SO3 groups in sulfonic acids. The same absorptions bands can be found in the IR spectra of the coatings applied to silicon substrates covered with gold (Fig. 5b). Of note is some displacement of the absorption bands to the lower wavenumbers. Thus, the stretching vibrations of SO3 groups typical for SPNBI appear at 1045 cm–1. This can result both from an extremely thin layer of the polymer on the substrate and due to the fact that, during electron beam dispersion, the most low-molecular fractions of the applied polymer samples are deposited first. Besides the bands at 1045 cm–1, the IR spectra of SPNBI and its salts also demonstrate absorption bands at ca. 1340 cm–1, which can be attributed to the stretching vibrations of SO2 groups in sulfones and appear, as it was already mentioned, due to desulfonation leading to the formation of intermolecular –SO2– bonds.

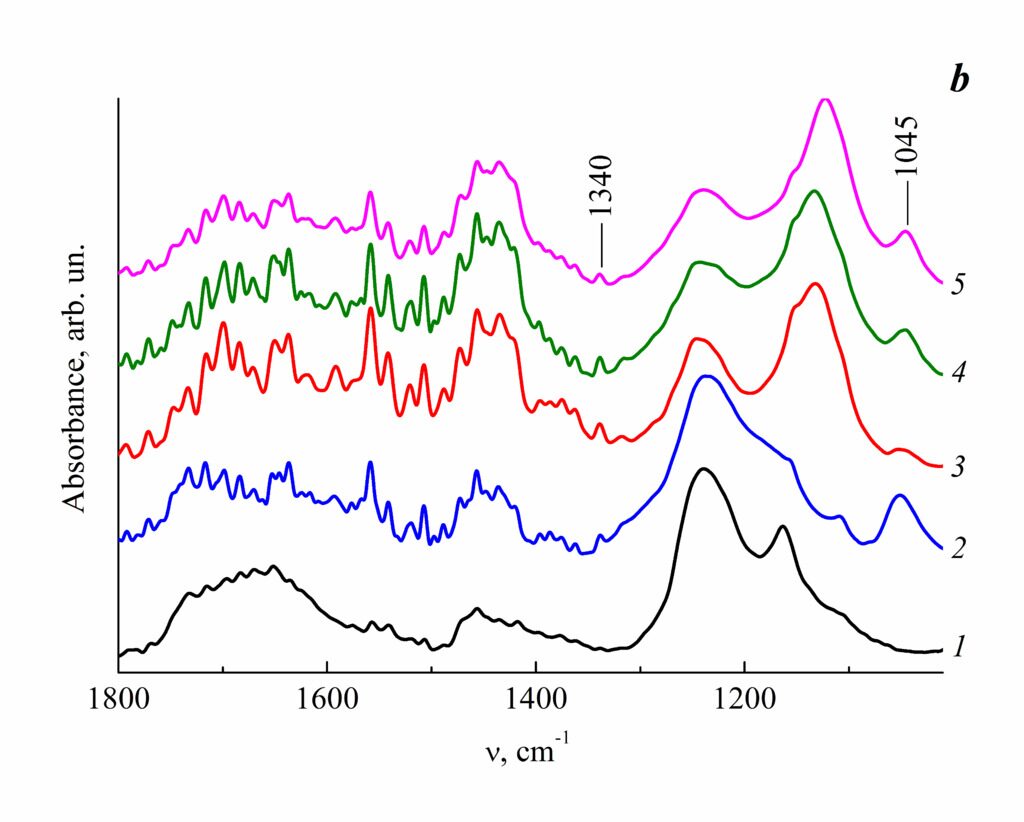
Figure 5. IR spectra of the initial powders (a) and the coatings on the silicon plates covered with gold (b) for PNBI (1), SPNBI-I,VI (2), SPNBI-I,VI-4.7%К (3), SPNBI-I,VI-2.5%Са (4), and SPNBI-I,VI-1.5%Zn (5). The metal contents are presented in wt %.
In the case of SPNBI bearing metal atoms, desulfonation occurs owing to the residual unsubstituted sulfo groups, which, as it was already mentioned, can remain in the solid salt forms. Note that the intensity of the band at 1045 cm–1 changes in the following series: SPNBI > SPNBI-Са » SPNBI-Zn > SPNBI-K. It cannot be excluded that desulfonation is a reason for the observed difference in the rate of film thickening in the case of PNBI, SPNBI and its metal derivatives during electron beam dispersion. Presumably, under action of an electron beam, the sample undergoes cross-linking and, as a consequence, the amount of the polymer, which converts to the gas phase, reduces; this is reflected in essentially differing thicknesses of the films at the same dispersion times.
We also measured the electrical conductivity of the applied coatings depending on the metal introduced into the polymer. The surface resistances of the coatings were measured on the substrates from single-crystalline silicon by double-probe method on an alternate current using an E7-20 LCR meter (Belarus) at the frequency of 1 kHz; the signal amplitude composed 1 V. The maximum value of the measured resistance was 10 GO. The distance between the probes on the surface was
Table 4. Surface resistance of the film samples of PNBI, SPNBI, and their salts
|
Coating composition |
PNBI-I,VI |
SPNBI-I,VI |
SPNBI-I,VI-1.5%Zna |
SPNBI-I,VI-4.7%Ka |
SPNBI-I,VI-2.5%Caa |
|
Resistance, GO/ |
>10 |
5 |
1 |
8 |
3 |
a the metal content is presented in wt %.
As it follows from Table 4, the highest conductivity is characteristic for the coating based on SPNBI-Zn and the lowest one—for the coating based on SPNBI-K.
The electrochemical sensor properties were studied by the example of potassium salt SPNBI-III,VI-1.5%K. The method of electron beam dispersion (as it was described above) was used to apply the polymer on a sitall plate with an interdigital system of electrodes. It was found that the sample under investigation possesses strong dielectric properties. During the impact, the polymer surface is charged and starts to mirror the primary electrons. As a result, these electrons affect the applied coating, which is partially cross-linked, presumably, due to the desulfonation processes. To provide a constant rate of the coating thickening, the electron current was controlled during the sample application. At the process beginning, it composed 5 mA, upon its completion—30 mA. The total time of dispersion was 70 min. According to the piezoelectric microweighing, the coating thickness was 20 nm. The electrodes were placed on the substrate using photolithography. The widths of the electrodes were 15 μm, the distance between the electrodes was 15 μm, their lengths were
Conclusions
To summarize the results presented, we synthesized new PNBIs and their sulfonated derivatives of various compositions. It was shown that the introduction of sulfo groups, on the one hand, improves the solubility of PNBIs and, on the other hand, opens the way to further modification by the introduction of metal ions into the structures of SPNBIs. The electrical properties of the coatings obtained from the metal derivatives of SPNBIs applied to the substrates were evaluated preliminarily by electron beam dispersion. This type of systems seems to be promising for the development of a new generation of biosensors—ion-sensitive electrochemical sensor coatings that would allow one to study the interaction of cells with the environment, in particular, to study the reactions of cells with drugs [13, 14].
Acknowledgements
This work was supported by the Ministry of Science and Higher Education of the Russian Federation.
References
- A. L. Rusanov, Russ. Chem. Rev., 1979, 48, 62–78. DOI: 10.1070/RC1979v048n01ABEH002303
- A. L. Rusanov, R. M. Kumykov, A. K. Mikitaev, New Soluble Thermo- and Fire-Resistant Polyheteroarylenes, Moscow, RKhTU im. D. I. Mendeleeva, 2007.
- A. L. Rusanov, S. N. Leont'eva, Ts. G. Iremashvili, Russ. Chem. Rev., 1977, 46, 76–91. DOI: 10.1070/RC1977v046n01ABEH002121
-
V. V. Korshak, A. L. Rusanov, A. M. Berlin, B. R. Livshits, T. Kh. Dymshits, L. N. Silyutina, V. F. Blinov, Polym. Sci. U.S.S.R., 1979, 21, 719–725. DOI: 10.1016/0032-3950(79)90300-9
- M. I. Buzin, V. G. Vasil'ev, G. G. Nikiforova, N. M. Belomoina, E. G. Bulycheva, V. S. Papkov, Dokl. Phys. Chem., 2014, 471, 195–198. DOI: 10.1134/S0012501616120010
- A. L. Rusanov, E. G. Bulycheva, G. S. Matvelashvili, G. V. Kazakova, Polym. Prepr., 1994, 35, 370.
- N. M. Belomoina, E. G. Bulycheva, A. L. Rusanov, J.-g. Liu, S.-y. Yang, Dokl. Chem., 2012, 444, 129–132. DOI: 10.1134/S0012500812060018
- N. G. Polyanskii, P. E. Tulupov, Russ. Chem. Rev., 1971, 40, 1030–1046. DOI: 10.1070/RC1971v040n12ABEH001990
- A. I. Egorov, V. P. Kazachenko, A. V. Rogachev, M Y. Yablokov, Russ. J. Phys. Chem., 2002, 76, 1898–1902.
- T. S. Demina, M. Yu. Yablokov, A. B. Gilman, A. I. Gaidar, T. A. Akopova, A. N. Zelenetskii, High Energy Chem., 2015, 49, 213–215. DOI: 10.1134/S0018143915030078
- L. I. Kravets, A. B. Gil'man, M. Yu. Yablokov, V. A. Altynov, O. L. Orelovitch, High Energy Chem., 2016, 50, 460–465. DOI: 10.1134/S0018143916060102
- L. I. Kravets, A. B. Gilman, M. Yu. Yablokov, A. N. Shchegolikhin, B. Mitu, G. Dinescu, High Temp. Mater. Processes, 2015, 19, 121–139. DOI: 10.1615/HighTempMatProc.2016016073
- Q. Liu, C. Wu, H. Cai, N. Hu, J. Zhou, P. Wang, Chem. Rev., 2014, 114, 6423–6461. DOI: 10.1021/cr2003129
- Electrochemical Sensor Analysis, S. Alegret, A. Merkoci (Eds.), in: Compr. Anal. Chem., Amsterdam, Elsevier, 2007, vol. 49.





