2019 Volume 2 Issue 4
|
|
INEOS OPEN, 2019, 2 (4), 130–133 Journal of Nesmeyanov Institute of Organoelement Compounds Download PDF DOI: 10.32931/io1918a |
|
4,7-Di-n-butoxy-1,10-phenanthroline-2,9-dicarboxamide: a Tetradentate Ligand Featuring Excellent Solubility in Nonpolar Media
a Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, Moscow, 119991 Russia
b Chemistry Department, Moscow State University, Leninskie Gory 1, str. 3, Moscow, 119991 Russia
Corresponding author: D. N. Zarubin, e-mail: zaroubine@ineos.ac.ru
Received 27 August 2019; accepted 27 September 2019
Abstract

A facile preparative method for introduction of lipophilic alkoxy groups at positions 4 and 7 of a phenanthroline core is developed. The syntheses of N,N'-diethyl-N,N'-di(4-n-hexylphenyl)-4,7-di-n-butoxy-1,10-phenanthroline-2,9-dicarbamide and its complex with lanthanum nitrate are described. A structure of the resulting complex is elucidated by XRD. A high solubility of the N,N',O,O'-tetradentate ligand in aliphatic hydrocarbons offers ample opportunities for its application for extraction of lanthanides and actinides into nonpolar organic solvents.
Key words: N,N',O,O'-tetradentate ligands, 1,10-phenanthroline-2,9-dicarboxylic acid amides, complexes of lanthanum nitrate, XRD.
Diamides of 1,10-phenanthroline-2,9-dicarboxylic acid (PDCA) and its derivatives comprise a large class of N,N',O,O'-tetradentate ligands that readily form complexes with transition and post-transition metal cations. Over recent years, they have assumed particular importance as promising extracting agents for processing of highly radioactive nuclear wastes [1–3]. Owing to the rigid prearranged structures, the presence of different coordination sites (hard amide oxygen and soft heterocyclic nitrogen atoms according to the Pearson's acid–base concept), and the high coordinating ability at relatively low basicity, these compounds can efficiently extract actinides and lanthanides from aqueous nitric acid solutions into organic solvents. Important advantages of PDCAs are high oxidative and hydrolytic stabilities. Furthermore, some of these compounds exhibited high selectivity factors for separation of An/Ln [2, 4–8] and Ln/Ln' [9] pairs. At the same time, the extensive use of PDCA in extraction technologies is seriously restricted by the tedious synthesis of compounds that have functional substituents in a phenanthroline core and, in some cases, also by their low stability under radiolysis conditions. For example, recent investigations revealed that N,N'-diethyl-N,Nʹ-di(4-n-hexyl-phenyl)-4,7-dichloro-1,10-phenanthroline-2,9-dicarboxamide [5, 9] (compound 1, Fig. 1), which showed high selectivity for separation of actinides and lanthanides, readily undergoes hydrolysis/alcoholysis in a water–octanol medium under action of γ-radiation [10]. An additional factor that restricts the use of PDCAs in extraction technologies is their low solubility in saturated hydrocarbons, which are widely used as the cheapest solvents with appropriate technological characteristics. Our studies [2, 3, 5, 9–11] and the results obtained by other research groups [1, 4, 6–8, 12] indicate that the introduction of substituents at different positions of PDCA and related N-donor heterocyclic ligands allows one to significantly vary their solubility and extracting properties. The introduction of lipophilic alkyl substituents СnH2n+1 and alkoxy groups ОСnH2n+1 (n ≥ 4) is often used to increase the solubility of organic compounds in nonpolar solvents. Recently, Borisova et al. [12] synthesized two PDCA derivatives with n-pentoxy groups at positions 4 and 7 of a phenanthroline core; however, no data were provided on their solubility in saturated hydrocarbons. In this report, we describe an alternative method for introduction of alkoxy groups at the mentioned positions of a phenanthroline core, which is independently developed by our research group and seems to be more facile than the previously reported approach [12] from the preparative point of view. Using the suggested method, we synthesized N,Nʹ-diethyl-N,Nʹ-di(4-n-hexylphenyl)-4,7-di-n-butoxy-1,10-phenanthroline-2,9-dicarboxamide (compound 2, Fig. 1) and its complex with lanthanum nitrate. A structure of the latter was elucidated by XRD.
Figure 1. 4,7-Disubstituted 1,10-phenanthroline-2,9-dicarboxamides.
The previously reported synthetic approach for the related diamide, namely, N,Nʹ-diethyl-N,Nʹ-diphenyl-4,7-di-n-pentoxy-1,10-phenanthroline-2,9-dicarboxamide was based on a nucleophilic substitution of the chlorine atoms in 4,7-dichloroneocuproine, which was accomplished under action of amyl alcohol in the presence of dimsyl sodium. Subsequent oxidation of 4,7-di-n-pentoxyneocuproine with selenium dioxide and nitric acid followed by the conventional conversion of resulting 4,7-di-n-pentoxyphenanthroline-2,9-dicarboxylic acid into the corresponding dicarboxamide allowed the authors to isolate the target product in 25% overall yield.
The approach suggested by our research group avoids a labor-consuming step of production of 4,7-dichloroneocuproine from known heterocyclic precursor 3 [13, 14] under action of a large excess of phosphoryl chloride. In our opinion, it seemed more rational to accomplish the direct О-alkylation of ambident dione 3 followed by the oxidation of resulting 4,7-di-n-alkoxyneocuproine 4 into dicarboxylic acid 6 via the intermediate formation of dicarboxaldehyde 5 (Scheme 1).
Scheme 1. Synthetic route to 4,7-di-n-butoxy-1,10-phenathroline-2,9-dicarboxamide 2 and its complex 8. Reagents and conditions: (i) nBuI, K2CO3, TEBAC, DMF, 80 °C, 12 h (76%); (ii) SeO2, 1,4-dioxane/H2O, D, 1.5 h (78%); (iii) H2O2, EtOH, r.t., 12 h (99%); (iv) SOCl2, DMF, r.t., 12 h; (v) 4-(EtNH)C6H4nHex, Et3N, THF, r.t., 12 h (64%); (vi) La(NO3)3(H2O)6, MeCN, D, 2 h (93%).
We found that the alkylation of both of the oxygen atoms in dione 3 with n-butyl iodide proceeds selectively in a polar aprotic medium and affords 4,7-di-n-butoxyneocuproine 4 in a good yield. Note that in this case potassium carbonate, utilized as a base, in the presence of a phase-transfer catalyst (TEBAC) provides the same efficiency as conventionally used, but less convenient sodium hydride [15–18]. The oxidation of 4 into dicarboxaldehyde 5 under action of selenium dioxide can be accomplished upon heating in a water–dioxane mixture. An attempt to convert 5 to 4,7-di-n-butoxy-1,10-phenanthroline-2,9-dicarboxylic acid 6 under action of nitric acid, which had been used in the synthesis of the related 1,10-phenanthroline-2,9-dicarboxylic acid [4, 19], led to the formation of a complex mixture of products. However, this oxidation can be carried out quantitatively upon treatment of dicarboxaldehyde 5 with hydrogen peroxide in ethanol at room temperature. Furthermore, compound 5 can be used without chromatographic purification from selenium-containing admixtures, since they do not interfere and, presumably, even catalyze the following oxidation. Interestingly, under these conditions, the heterocyclic nitrogen atoms remain intact, allowing one to avoid the potential formation of N-oxides. The conversion of dicarboxylic acid 6 to dicarboxamide 2 was carried out by the conventional method via intermediate generation of dichloride 7. Product 2 was isolated as a viscous oil and was characterized by IR and NMR spectra. As it was expected, it readily mixes with aliphatic, aromatic and chlorine-substituted hydrocarbons almost in any ratio.
The 1Н NMR spectrum of diamide 2 exhibits pronounced temperature dependence. The signals in the spectrum of a solution of 2 in deuterotoluene registered at room temperature are very broad (Fig. 2а). A temperature rise to 80 °С leads to the narrowing of the signals, which acquire the expected multiplicities (Fig. 2b). This type of a temperature dependence was already observed in the spectra of PDCA [6, 9] and the related diamides of pyridine-2,6-dicarboxylic acid [11]. In particular, they are observed in the 1H NMR spectrum of diamide 1 [9]. These effects stem from the formation of diamide associates in solutions, which decompose with a temperature rise. This association is supposed to stem from π-stacking of PDCA molecules, although there is no strong evidence. For cyclic dilactams derived from pyridine-2,6-dicarboxylic acid, π-stacking was detected in crystals [11].

Figure 2. Aromatic part of the 1Н NMR spectrum of 0.05 M solution of 4,7-di-n-butoxy-N,N'-diethyl-N,N'-di(4-n-hexylphenyl)-1,10-phenanthroline-2,9-dicarboxamide 2 in toluene-d8 at 19 °C (a) and 80 °C (b). Asterisks (*) indicate residual proton signals of the solvent.
Heating of equimolar amounts of 2 and La(NO3)3(H2O)6 in acetonitrile afforded complex 8 with 1:1 composition in a yield close to the quantitative one (Scheme 1). The resulting complex is moderately soluble in acetonitrile and is readily crystallized at room temperature as pale-yellow polyhedral crystals. The 1Н and 13С{1Н} NMR spectra recorded at room temperature show a characteristic set of narrow singlets and multiplets with well-resolved fine structures. This evidences the retention of an initial planar symmetry of the ligand after binding with the metal ion.
The IR spectrum of the complex contains absorption bands characteristic for the stretching vibrations of nitrate ions coordinated by the metal ions, which are manifested as two very intensive bands at 1467 and 1274 cm–1. The absorption band associated with the C=O bond stretching vibrations is detected at 1615 cm–1 and is shifted to the lower frequencies compared to the spectrum of the corresponding dicarboxamide, where it appears at 1650 cm–1. This is typical for coordination by the metal ion. The molecular structure of complex 8 obtained by XRD is depicted in Fig. 3.
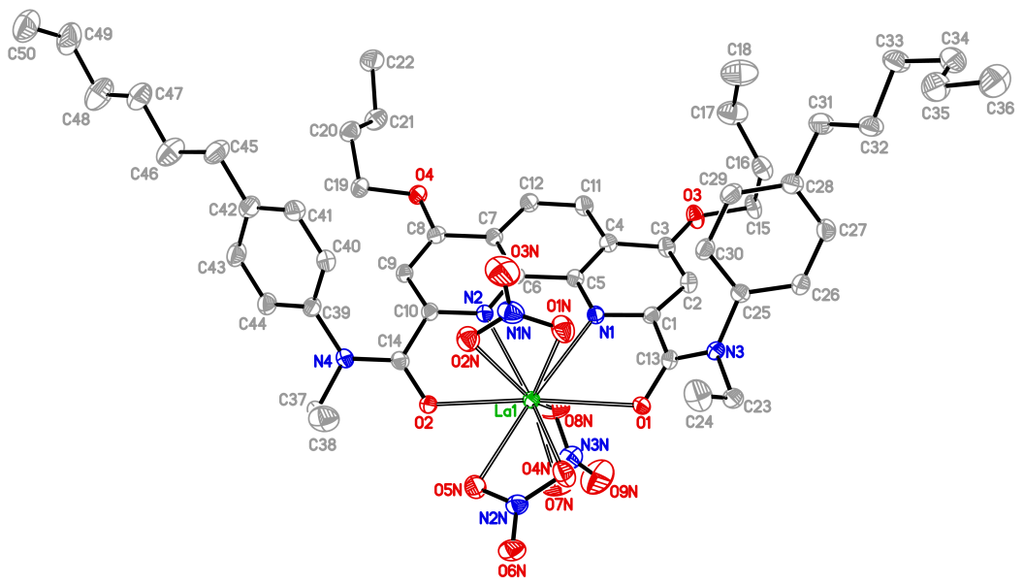
Figure 3. Molecular structure of complex 8 (thermal ellipsoids are presented at 50% probability level; the hydrogen atoms are omitted for clarity). Selected bond distances (Å) and angles (deg) La(1)–N(1) 2.6899(18), La(1)–N(2) 2.6990(17), La(1)–O(1) 2.5689(15), La(1)–O(2) 2.5314(15), La(1)–O(1N) 2.6078(17), La(1)–O(2N) 2.6166(17), La(1)–O(4N) 2.6321(17), La(1)–O(5N) 2.6484(17), La(1)–O(7N) 2.5661(18), La(1)–O(8N) 2.5808(18), O(2)–La(1)–O(1) 168.54(5), N(1)–La(1)–N(2) 59.49(5).
In a coordination sphere of the lanthanum ion (CN = 10), tetradentate ligand 2 is almost symmetrically bound via two oxygen atoms of the amide groups and two nitrogen atoms of the phenanthroline moiety. The nitrate anions serve as bidentate ligands. The more bulky aryl substituents at the amide nitrogen atoms adopt anti-positions relative to the metal ion. O(1) and O(2) amide oxygen atoms significantly deviate from a mean plane of the phenanthroline ligand towards the metal ion (the corresponding distances compose 0.53 and 0.88 Å, respectively). Consequently, the metal atom appeared to be below the same plane by 0.45 Å. A reason for these deviations, which are typical for PDCA complexes with lanthanides and actinides, was elucidated earlier by our research group upon quantum chemical investigation of structures of the related complexes [9, 20]. The gas-phase calculations showed that winding of the amide groups towards the metal ion interrupts their conjugation with the electron-deficient phenanthroline core, which leads to an increase in the negative charge on the oxygen atoms. The metal–oxygen coordination bond has predominantly an ionic electrostatic nature; therefore, this winding affords its strengthening and general stabilization of the complex. This structural effect was also detected for the complexes of pyridine-2,6-dicarboxylic acid diamides with lanthanides and actinides [3, 20, 21].
In crystals, the molecules of complex 8 are assembled into centrosymmetric dimers (Electronic supplementary information (ESI), Fig. S1) with an insignificant overlapping of polycyclic systems of the phenanthroline ligands.
In general, the structure of compound 8 is analogous to those of the previously described complexes (PDCA)M(NO3)3 (M = La, Eu [12], Nd, and Gd [6]) and the geometry around the metal center is close to those of the above-mentioned complexes and [(PDCA)2La(NO3)2](NO3) [6] (ESI, Table S1).
Hence, in this report we suggested a facile method for introduction of alkoxy groups at positions 4 and 7 of a phenanthroline core, which opens the way to a wide range of new functionalized phenanthroline derivatives, including diamides of 4,7-dialkoxy-1,10-phenanthroline-2,9-dicarboxylic acid. We synthesized N,Nʹ-diethyl-N,Nʹ-di(4-n-hexylphenyl)-4,7-di-n-butoxy-1,10-phenanthroline-2,9-dicarboxamide 2 that features a higher solubility in aliphatic hydrocarbons than other PDCA derivatives, which are deprived of lipophilic groups at positions 4 and 7. The investigation of extracting properties of compound 2 is currently under way in our laboratory, and the results obtained will be published in due course.
Acknowledgements
This work was supported by the National Intellectual Development Foundation (Innopraktika; contract no. 1315 (2017)) and the Ministry of Science and Higher Education of the Russian Federation.
The authors are grateful to the Center for Molecular Composition Studies of INEOS RAS for investigation of the compounds structures.
Electronic supplementary information
Electronic supplementary information (ESI) available online: synthetic procedures, X-ray data for complex 8. For ESI, see DOI: 10.32931/io1918a
References
- D. Merrill, R. D. Hancock, Radiochim. Acta, 2011, 99, 161–166. DOI: 10.1524/ract.2011.1805
- M. Yu. Alyapyshev, V. A. Babain, Yu. A. Ustynyuk, Russ. Chem. Rev., 2016, 85, 943–961. DOI: 10.1070/rcr4589
- Yu. A. Ustynyuk, M. Yu. Alyapyshev, V. A. Babain, N. A. Ustynyuk, Russ. Chem. Rev., 2016, 85, 917–942. DOI: 10.1070/rcr4588
- C.-L. Xiao, C.-Z. Wang, L.-Y. Yuan, B. Li, H. He, S. Wang, Y.-L. Zhao, Z.-F. Chai, W.-Q. Shi, Inorg. Chem., 2014, 53, 1712–1720. DOI: 10.1021/ic402784c
- M. Alyapyshev, V. Babain, L. Tkachenko, E. Kenf, I. Voronaev, D. Dar'in, P. Matveev, V. Petrov, S. Kalmykov, Y. Ustynyuk, J. Radioanal. Nucl. Chem., 2018, 316, 419–428. DOI: 10.1007/s10967-018-5775-7
- M. Alyapyshev, J. Ashina, D. Dar'in, E. Kenf, D. Kirsanov, L. Tkachenko, A. Legin, G. Starova, V. Babain, RSC Adv., 2016, 6, 68642–68652. DOI: 10.1039/c6ra08946a
- M. Galletta, S. Scaravaggi, E. Macerata, A. Famulari, A. Mele, W. Panzeri, F. Sansone, A. Casnati, M. Mariani, Dalton Trans., 2013, 42, 16930–16938. DOI: 10.1039/c3dt52104d
- J. Dehaudt, N. J. Williams, I. A. Shkrob, H. Luo, S. Dai, Dalton Trans., 2016, 45, 11624–11627. DOI: 10.1039/c6dt01800a
- Yu. A. Ustynyuk, N. E. Borisova, V. A. Babain, I. P. Gloriozov, A. Y. Manuilov, S. N. Kalmykov, M. Yu. Alyapyshev, L. I. Tkachenko, E. V. Kenf, N. A. Ustynyuk, Chem. Commun., 2015, 51, 7466–7469. DOI: 10.1039/c5cc01620g
- 1P. I. Matveev, A. A. Mitrofanov, V. G. Petrov, S. S. Zhokhov, A. A. Smirnova, Yu. A. Ustynyuk, S. N. Kalmykov, RSC Adv., 2017, 7, 55441–55449. DOI: 10.1039/c7ra11622e
- H. V. Lavrov, N. A. Ustynyuk, P. I. Matveev, I. P. Gloriozov, S. S. Zhokhov, M. Yu. Alyapyshev, L. I. Tkachenko, L. G. Voronaev, V. A. Babain, S. N. Kalmykov, Yu. A. Ustynyuk, Dalton Trans., 2017, 46, 10926–10934. DOI: 10.1039/c7dt01009e
- N. E. Borisova, A. A. Kostin, M. D. Reshetova, K. A. Lyssenko, E. V. Belova, B. F. Myasoedov, Inorg. Chim. Acta, 2018, 478, 148–154. DOI: 10.1016/j.ica.2018.03.042
- M. Schmittel, H. Ammon, Eur. J. Org. Chem., 1998, 785–792. DOI: 10.1002/(SICI)1099-0690(199805)1998:5<785::AID-EJOC785>3.0.CO;2-%23
- A. F. Larsen, T. Ulven, Org. Lett., 2011, 13, 3546–3548. DOI: 10.1021/ol201321z
- S. Strömberg, M. Oksman, L. Zhang, K. Zetterberg, Acta Chem. Scand., 1995, 49, 689–695. DOI: 10.3891/acta.chem.scand.49-0689
- H. Frisell, B. Aekermark, Organometallics, 1995, 14, 561–563. DOI: 10.1021/om00001a078
- G. K.-M. So, G. Cheng, J. Wang, X. Chang, C.-C. Kwok, H. Zhang, C.-M. Che, Chem. Asian J., 2017, 12, 1490–1498. DOI: 10.1002/asia.201700081
- X. Liu, X. Li, Y. Chen, Y. Hu, Y. Kishi, J. Am. Chem. Soc., 2012, 134, 6136–6139. DOI: 10.1021/ja302177z
- L. Gude, M.-J. Fernández, K. B. Grant, A. Lorente, Org. Biomol. Chem., 2005, 3, 1856–1862. DOI: 10.1039/b502485d
- M. Alyapyshev, V. Babain, L. Tkachenko, V. Gurzhiy, A. Zolotarev, Y. Ustynyuk, I. Gloriozov, A. Lumpov, D. Dar'in, A. Paulenova, Z. Anorg. Allg. Chem., 2017, 643, 585–592. DOI: 10.1002/zaac.201700063
- Yu. A. Ustynyuk, I. P. Gloriozov, S. N. Kalmykov, A. A. Mitrofanov, V. A. Babain, M. Yu. Alyapyshev, N. A. Ustynyuk, Solvent Extr. Ion Exch., 2014, 32, 508–528. DOI: 10.1080/07366299.2014.915666





