2018 Volume 1 Issue 2
|
|
INEOS OPEN, 2018, 1(2), 98–102 Journal of Nesmeyanov Institute of Organoelement Compounds DOI: 10.32931/io1809a |
|
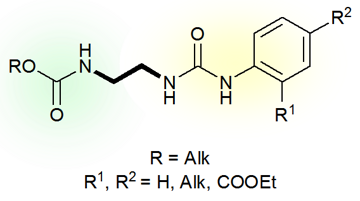
N-Alkoxycarbonylaminoethyl-N'-arylureas Exhibiting Antistress and Plant Growth Regulation Properties Under Unfavorable Environmental Conditions
I. N. Solovieva,a M. M. Vorob'ev,b and K. A. Kochetkov*a,b
a Mendeleev University of Chemical Technology of Russia, Miusskaya pl. 9, Moscow, 125047 Russia
b Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, Moscow, 119991 Russia
Corresponding author: K. A. Kochetkov, e-mail: const@ineos.ac.ru
Received 28 May 2018; accepted 11 September 2018
Abstract
A series of new (alkoxycarbonylaminoethyl)arylureas are synthesized and tested for the plant growth regulation activity in plants and plant cell cultures. The compounds obtained exert a significant effect on metabolic processes in plant cells, stimulating cell growth and, concurrently, considerably inhibiting or stimulating metabolic processes related to cell respiration. An efficient plant growth regulation activity exhibited by these derivatives can ensure plant resistance to unfavorable environmental conditions.
Key words: arylureas, cytokinins, plant growth regulation activity, seed treatment.
Introduction
Recent years have witnessed an extensive search for compounds exhibiting herbicidal properties. The new concepts have been suggested for creating original complex preparative forms of herbicides. The adverse environmental effects of a new generation of herbicidal preparations have been evaluated, and the methods for removing their impact have been elaborated. The toxicities of herbicides and their decomposition products towards a variety of biological species have been assessed [1–5]. Nowadays, of particular interest are investigations concerned with different growth regulators that can mitigate phytotoxic effects of herbicides [6–12].
There are numerous experimental data on the adaptogenic impact of different plant growth regulators on the herbicidal effect. The application of plant growth regulators both for pre-seeding treatment and in conjunction with herbicides showed a lot of promise for improving the productivity of cultivated plants [13]. Plant hormones (phytohormones) or their synthetic analogs, in particular, cytokinins are increasingly used as plant growth regulators [14, 15].
Cytokinins are involved in regulation of many physiological processes in plants, such as prevention of wilting, mobilization of nutrients, stem growth, formation and activity of an apical meristem of shoots, germination of seeds, etc. [16–18].
Plant growth regulators showing cytokinin activity include a number of carbamate derivatives, such as kartolin, phenmedipham, EDU, and kartolin-2 (Fig. 1) [19–22], which are effective in extremely low doses. Cytokinins take part in regulation of many physiological processes of plants.
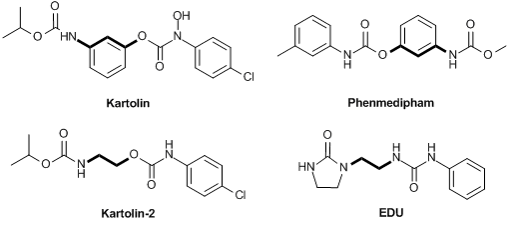
Figure 1. Structures of synthetic analogs of cytokinins.
Results and discussion
The structures of synthetic analogs of cytokinins include two toxophores connected by an alkyl chain. Comparing the structures of kartolin-2 and EDU, it can be assumed that the compounds bearing different toxophores, namely, carbamate and carbamide groups separated by an ethylene bridge can also exhibit plant growth regulation activity. Taking this into account, we synthesized a series of bifunctional compounds based on ethylenediamine, in which the carbamate and urea toxophores are connected by an ethylene bridge, and studied their phytoactivity.
The target compounds were obtained upon treatment of β-aminoethylcarbamic acid esters 1–3 with aromatic isocyanates. In turn, esters 1–3 were synthesized by the acylation of ethylenediamine with alkyl chloroformates (Scheme 1).
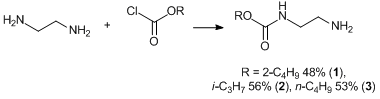
Scheme 1. Synthesis of β-aminoethylcarbamic acid esters 1–3.
The reactions of N-(2-aminoethyl)alkylcarbamates 1–3 with aromatic isocyanates in toluene led to the desired carbaminoethylene-substituted ureas 4–10 in high yields (87–95%) (Scheme 2).
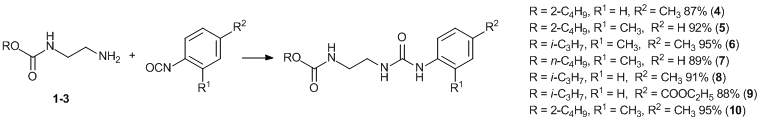
Scheme 2. Synthesis of N-alkoxycarbonylaminoethyl-N'-arylureas 4–10.
The compounds obtained are air-stable crystalline solids featuring high melting points (160–200 °С). They are well soluble in ethanol and polar organic solvents. The investigation of their activity towards tobacco cell culture, grown under strictly controlled conditions, revealed their significant effect on metabolic processes in plant cells (Table 1). They stimulated cell growth along with significant inhibition or stimulation of metabolic processes related to cell respiration.
Table 1. Biological activity of the compounds obtained towards
tobacco cell culture
|
Compound |
Intensity of cell growth (slurry density, d), |
Specific respiration intensity (CO2/d), |
|
4 |
195.1 ± 2.0 |
109.0 ± 0.8 |
|
5 |
180.2 ± 1.0 |
136.2 ± 1.1 |
|
6 |
145.2 ± 1.2 |
29.5 ± 0.3 |
|
7 |
173.1 ± 1.3 |
128.7 ± 1.1 |
|
8 |
159.6 ± 1.2 |
128.4 ± 1.1 |
|
9 |
94.9 ± 1.1 |
250.1 ± 2.1 |
|
10 |
114.2 ± 1.0 |
54.6 ± 0.5 |
|
Kartolin-2 |
152.3 ± 1.1 |
63.2 ± 0.5 |
|
control |
100.1 ± 1.0 |
100.1 ± 1.1 |
Whole plant tests confirmed the growth regulation activity of compounds 8 and 9 (Table 2). Thus, the compounds explored are prone to inhibit the growth of vegetative organs of both mono- and dicotyledonous plants. This effect corresponds to antistress activity, since the inhibition of growth appears to be a defensive response to unfavorable external conditions. Growth inhibitor maleic acid hydrazide (MAH) was used as a reference, whereas kartolin-2 was inactive.
Table 2. Effect of compounds 8 and 9 on growth of vegetative organs of beans and oats
|
Compound |
Plant weight, % of control |
|
|
Bean |
Oat |
|
|
8 |
41.6 ± 0.2 |
65.9 ± 0.3 |
|
9 |
62.3 ± 0.3 |
61.7 ± 0.3 |
|
MAH |
26.1 ± 0.2 |
107.2 ± 1.1 |
In drought resistance tests on Moskovskaya 35 spring wheat, the following parameters were evaluated: (1) water retention capacity of sprout tissues, which is determined by the loss of water upon drying; (2) the number of survived plants upon dehydration (the same extent in all variants); (3) dry mass of plants after regeneration in a humid chamber, which characterizes biochemical resistance to dehydration—the higher the resulting value, the higher the resistance. Chlorocholine chloride (drought resistance-increasing agent) was used as a reference to compare the activities achieved in different experiments. Kartolin-2 showed the reduced activity. The results of investigations on biological activity of compounds 4–8 and 10 relative to that of chlorocholine chloride are presented in Table 3. As can be seen, compounds 4–8, 10 showed higher activities than kartolin-2 and chlorocholine chloride, which may positively affect the drought resistance of plants.
Table 3. Increase in the activity of compounds 4–8 and 10 relative to that of chlorocholine chloride
|
Compound |
C, mg/L |
Drought resistance |
||
|
Water |
Survival rate, % of control |
Dry mass, mg/plant |
||
|
4 |
10 |
7.1 ± 0.2 |
21.3 ± 0.2 |
0.51 ± 0.03 |
|
1 |
5.0 ± 0.1 |
27.4 ± 0.1 |
1.12 ± 0.02 |
|
|
0.1 |
5.7 ± 0.2 |
33.8 ± 0.2 |
1.98 ± 0.01 |
|
|
5 |
10 |
5.0 ± 0.2 |
37.3 ± 0.2 |
0.74 ± 0.01 |
|
1 |
3.0 ± 0.1 |
21.1 ± 0.1 |
1.40 ± 0.02 |
|
|
0.1 |
3.2 ± 0.1 |
45.7 ± 0.3 |
1.08 ± 0.01 |
|
|
6 |
10 |
4.1 ± 0.2 |
73.6 ± 0.4 |
0.01 ± 0.01 |
|
1 |
1.3 ± 0.1 |
109.5 ± 0.5 |
0.01 ± 0.01 |
|
|
0.1 |
1.4 ± 0.2 |
45.4 ± 0.4 |
0.43 ± 0.01 |
|
|
7 |
10 |
2.1 ± 0.1 |
117.3 ± 0.6 |
0.01 ± 0.01 |
|
1 |
1.2 ± 0.2 |
45.2 ± 0.2 |
0.01 ± 0.01 |
|
|
0.1 |
7.3 ± 0.2 |
81.4 ± 0.3 |
0.32 ± 0.01 |
|
|
8 |
10 |
2.1 ± 0.1 |
30.1 ± 0.2 |
1.06 ± 0.03 |
|
1 |
2.3 ± 0.2 |
–8.1 ± 0.1 |
–0.58 ± 0.02 |
|
|
0.1 |
3.4 ± 0.2 |
21.8 ± 0.2 |
–0.35 ± 0.02 |
|
|
10 |
10 |
7.1 ± 0.4 |
4.5 ± 0.1 |
–0.75 ± 0.03 |
|
1 |
0.20 ± 0.01 |
49.4 ± 0.2 |
0.54 ± 0.02 |
|
|
0.1 |
2.1 ± 0.1 |
21.6 ± 0.2 |
0.51 ± 0.02 |
|
|
Kartolin-2 |
10 |
Not active |
–33.3 ± 0.2 |
Not active |
|
1 |
20.1 ± 0.2 |
|||
|
0.1 |
–22.3 ± 0.2 |
|||
Experimental
General remarks
All the reagents and solvents were obtained from commercial sources and used without further purification. Structures of all the compounds derived were confirmed based on the data of 1H and 13C NMR spectroscopy, mass spectrometry and elemental analyses. The 1Н and 13С NMR spectra were recorded on a Bruker DRX-400 spectrometer operating at the frequency of 400.13 MHz, using CDCl3 as a solvent and TMS as an internal standard. The chemical shifts were measured with 0.01 ppm accuracy; the coupling constants are reported in Hertz quoted to the nearest 0.1 Hz. The mass spectra were recorded on an Agilent 1100 Series spectrometer in positive ion-detection mode. Direct injection of the analyzed solution was used. The flow rate composed 400 µL/h. The temperature of a desiccative gas was 350 °С. The gas pressure composed 10 psi. The voltage of a needle nebulizer ranged within 4.5–5.5 kV. The isotope distribution was calculated using Molecular Weight Calculator software, version 6.73. The elemental analyses were carried out at the Laboratory of Microanalysis, INEOS RAS. The melting points were measured with a Meltemp II instrument. The IR spectra were recorded on a Nicolet Magna-IR 750 FT-spectrometer at the Centre for Joint Use of Mendeleev University (KBr pellets).
Syntheses
General procedure for the synthesis of β-aminoethylcarbamic acid esters 1–3 (for the published procedure, see ref. [23]).
Esters 1–3 were obtained by the reactions of alkyl chloroformates with ethylenediamine using the following procedure. 100 mL of the corresponding alcohol was placed into a 250-mL three-neck flask, equipped with a magnetic stirrer, a thermometer, a reflux condenser with an absorber of HCl and a bubbler, connected to a phosgene line through the system of sequentially connected flasks containing air and concentrated sulfuric acid. Phosgene was bubbled through the alcohol at 0 °С until the formation of HCl had ceased. Then the reaction mixture was poured onto ice and extracted with ethyl ether. The organic layer was dried over Na2SO4 and concentrated on a rotary evaporator. The residue was purified by vacuum distillation. For the synthesis of β-aminoethylcarbamic acid esters, the corresponding alkyl chloroformate was added dropwise to a vigorously stirred solution of ethylenediamine (10-fold excess) in dry toluene at 0–5 °С. The reaction mixture was stirred at room temperature for 2 hours. The resulting precipitate was filtered off, and the filtrate was washed with brine (3 times). The organic layer was separated, dried over anhydrous Na2SO4, and concentrated on a rotary evaporator. The excess of ethylenediamine was removed by distillation in vacuum. The desired β-aminoethylcarbamic acid ester was purified by vacuum distillation.
2-Aminoethylcarbamic acid sec-butyl ester, 1. Yield: 48%. Bp: 154–155 °C/0.1 mm Hg (compare with 155–156 °C/0.1 mm Hg in ref. [24]).
2-Aminoethylcarbamic acid iso-propyl ester, 2. Yield: 56%. Bp: 148–153 °C/0.1 mm Hg (compare with 146–154 °C/0.1 mm Hg in ref. [24]).
2-Aminoethylcarbamic acid butyl ester, 3. Yield: 53%. Bp: 160–162 °C/0.1 mm Hg (compare with 160–163 °C/0.1 mm Hg in ref. [24]).
General procedure for the synthesis of N-alkoxycarbonylaminoethyl-N'-arylureas 4–10.
The corresponding aryl isocyanate (10 mmol) was added to a stirred solution of β-aminoethylcarbamic acid ester 1, 2, or 3 (10 mmol) in 15 mL of dry toluene. In 30 min, the resulting precipitate was filtered off, recrystallized from isopropanol and dried.
N'-(4-Tolyl)-N-[2-(sec-buthoxycarbonylamino)ethyl]urea, 4. Yield: 87%. Mp: 162–164 °С. Anal. Calcd for С15Н23N3O3: С, 61.41; Н, 7.90; N, 14.32. Found: С, 61.62; Н, 7.88; N, 14.45%. 1H NMR (CDCl3, 25 °С, δ, ppm, J/Hz): 0.85 (t, 3Н, СН3-СН2 (2-Bu), J = 7.3), 1.14 (d, 3Н, СН3-СН (2-Bu), J = 5.7), 1.41–1.59 (m, 2Н, СН2 (2-Bu)), 2.28 (s, 3Н, СН3-Ar), 3.21–3.39 (m, 4H, CH2NH), 4.63 (m, 1H, CH (2-Bu)), 7.04 and 7.19 (АB, 4Н, HAr, J = 7.0). 13C{1H} NMR (CDCl3, 25 °С, δ, ppm, J/Hz): 9.29 (CH3CH2 (2-Bu)), 18.80 (СН3CH (2-Bu)), 21.00 (CH3-Ar), 28.58 (СH2 (2-Bu)), 38.63 and 43.63 (both CH2NH), 72.21 (CH (2-Bu)), 126.00, 130.55, 135.52, 136.70, 151.74 (NHC(O)NH), 152.80 (NHC(O)O). IR (ν/сm–1): 1239 (СОС), 1558–1607 (Ar), 1679 and 1697 (С=О).
N-(2-Tolyl)-N'-[2-(sec-butoxycarbonylamino)ethyl]urea, 5. Yield: 92%. Mp: 161–163 °С. Anal. Calcd for С15Н23N3O3: С, 61.41; Н, 7.90; N, 14.32. Found: С, 61.43; Н, 7.89; N, 14.44%. 1H NMR (CDCl3, 25 °C, δ, ppm, J/Hz): 0.85 (t, 3Н, СН3-СН2 (2-Bu), J = 7.4), 1.14 (d, 3Н, СН3-СН (2-Bu), J = 6.2), 1.42–1.57 (m, 2Н, СН2 (2-Bu)), 2.24 (s, 3Н, СН3-Ar), 3.21–3.35 (m, 4H, CH2NH), 4.63 (m, 1H, CH (2-Bu)), 7.09 and 7.38 (АB, 4Н, HAr, J = 7.3). 13C{1H} NMR (CDCl3, 25 °С, δ, ppm, J/Hz): 9.29 (CH3CH2 (2-Bu)), 17.80 (CH3-Ar), 18.80 (СН3CH (2-Bu)), 28.58 (СH2 (2-Bu)), 39.80 and 41.83 (both CH2NH), 72.21 (CH (2-Bu)), 126.00, 126.04, 127.20, 131.10, 133.24, 136.41, 151.80 (NHC(O)NH), 154.43 (NHC(O)O). IR (ν/сm–1): 1240 (СОС), 1560–1610 (Ar), 1640 and 1701 (С=О).
N-2,4-Dimethylphenyl-N'-[2-(isopropoxycarbonylamino)-ethyl]urea, 6. Yield: 95%. Mp: 197–198 °С. Anal. Calcd for С15Н23N3O3: С, 61.41; Н, 7.90; N, 14.32. Found: С, 61.49; Н, 7.80; N, 14.38%. 1H NMR (DMSO-d6, 25 °С, δ, ppm, J/Hz): 1.16 (d, 6Н, СН3 (i-Pr), J = 6.4), 2.13 (s, 3Н, p-СН3-Ar), 2.20 (s, 3Н, o-СН3-Ar), 3.02–3.18 (m, 4H, CH2NH), 3.45 (s, 1Н, NHC(O)O), 4.76 (m, 1H, CH (i-Pr)), 6.50 (s, 1Н, NHC(O)NHAr), 6.85–6.96 (s, 1Н, HAr), 7.03 (m, 1Н, NHAr), 7.54–7.67 (m, 2Н, HAr). 13С{1H} NMR (DMSO-d6, 25°C, δ, ppm): 18.30, 20.82, 22.50, 39.47, 41.30, 67.13 (СH (i-Pr)), 121.70, 126.92, 127.91, 129.50, 131.10, 131.38, 135.92, 156.20 (NHC(O)NH), 157.4 (NHC(O)O). IR (ν/сm–1): 1235 (СОС), 1558–1607 (Ar), 1635 and 1691 (С=О).
N-(2-Tolyl)-N'-[2-(n-butoxycarbonylamino)ethyl]urea, 7. Yield: 89%. Mp: 161–163 °С. Anal. Calcd for С15Н23N3O3: С, 61.41; Н, 7.90; N, 14.32. Found: С, 61.40; Н, 7.82; N, 14.34%. 1H NMR (CDCl3, 25 °С, δ, ppm, J/Hz): 0.85 (t, 3Н, СН3 (n-Bu), J = 7.4), 1.44 (m, 2Н, СН3СН2 (n-Bu)), 1.64 (m, 2Н, СН2СН2O (n-Bu)), 2.24 (s, 3Н, СН3-Ar), 3.21–3.35 (m, 4H, CH2NH), 4.16 (t, 2H, CH2O (n-Bu), J = 6.6), 7.09 and 7.38 (АB, 4Н, HAr, J = 7.5). 13C{1H} NMR (CDCl3, 25 °С, δ, ppm, J/Hz): 13.65 (СН3 (Bu)), 17.80 (CH3-Ar), 19.20, 30.62, 39.80 and 41.83 (both CH2NH), 66.61 (CH2O (n-Bu)), 126.00, 126.04, 127.20, 131.10, 133.24, 136.41, 151.80 (NHC(O)NH), 153.14 (NHC(O)O). IR (ν/сm–1): 1244 (СОС), 1558–1607 (Ar), 1681 and 1741 (С=О).
N-(4-Tolyl)-N'-[2-(isopropoxycarbonylamino)ethyl]urea, 8. Yield: 98%. Mp: 183–184 °С. Anal. Calcd for C14H21N3O3: С, 61.20; Н, 7.58; N, 15.04. Found: С, 60.34; Н, 7.66; N, 14.98%. 1H NMR (CDCl3, 25 °С, δ, ppm, J/Hz): 1.16 (d, 6Н, СН3 (i-Pr), J = 6.4), 2.28 (s, 3Н, СН3-Ar), 3.21–3.39 (m, 4H, CH2NH), 4.76 (m, 1H, CH (i-Pr)), 7.04 and 7.19 (АB, 4Н, HAr, J = 7.0). 13C{1H} NMR (DMSO-d6, 25 °С, δ, ppm): 18.70 (CH3-Ar), 20.70 (CH3 (i-Pr)), 38.08 and 41.42 (both CH2NH), 67.21 (CH (i-Pr)), 121.34, 129.07, 132.70, 136.64, 150.08 (NHC(O)NH), 156.44 (NHC(O)O). IR (ν/сm–1): 1235 (СОС), 1558–1607 (Ar), 1635 and 1691 (С=О).
N-(4-Ethoxycarbonylphenyl)-N'-[2-(isopropoxycarbonyl-amino)ethyl]urea, 9. Yield: 88%. Mp: 165–167 °С. Anal. Calcd for С16Н23N3O5: С, 56.96; Н, 6.87; N, 12.45. Found: С, 57.04; Н, 6.94; N, 12.49%. 1H NMR (CDCl3, 25 °С, δ, ppm, J/Hz): 1.16 (d, 6Н, СН3 (i-Pr), J = 6.4), 1.24 (t, 3Н, СH3 (Et), J = 7.6), 3.21–3.39 (m, 4H, CH2NH), 4.31 (q, 2Н, CH2 (Et), J = 7.6), 4.76 (m, 1H, CH (i-Pr)), 7.04 and 7.19 (АB, 4Н, HAr, J = 7.1). 13C{1H} NMR (CDCl3, 25 °С, δ, ppm, J/Hz): 14.33, 19.68, 40.30 and 41.36 (both CH2NH), 60.78 (СН2 (Et)), 68.50 (СН (i-Pr)), 118.28, 124.45, 130.79, 143.25, 156.27 (NHC(O)NH), 157.77 (NHC(O)O), 166.4 (C(O)OEt). IR (ν/сm–1): 1233 (СОС), 1555–1606 (Ar), 1632 and 1690 (С=О).
N-(2,4-dimethylphenyl)-N'-[2-(sec-butoxycarbonylamino)-ethyl]urea, 10. Yield: 95%. Mp: 162–164 °С. Anal. Calcd for: С16Н25N3O3: С, 62.52; Н, 8.20; N, 13.67. Found: С, 62.57; Н, 8.28; N, 13.45%. 1H NMR (CDCl3, 25 °С, δ, ppm, J/Hz): 0.85 (t, 3Н, СН3-СН2 (2-Bu), J = 7.3), 1.14 (d, 3Н, СН3-СН (2-Bu), J = 6.4), 1.41–1.59 (m, 2Н, СН2 (2-Bu)), 2.21 (s, 3H, o-CH3-Ar), 2.28 (s, 3Н, p-СН3-Ar), 3.21–3.39 (m, 4H, CH2NH), 4.63 (m, 1H, CH (2-Bu)), 6.98 (d, 1Н, HAr, J = 8.8), 7.02 (s, 1H, HAr), 7.17 (d, 1H, HAr, J = 8.8). 13С{1H} NMR (DMSO-d6, 25 °C, δ, ppm): 9.29, 18.30, 18.80, 20.80, 28.58, 39.50 and 41.30 (both CH2NH), 72.21 (CH (2-Bu)), 121.72, 126.91, 127.89, 129.52, 131.43, 135.90, 156.20 (NHC(O)NH), 157.40 (NHC(O)O). IR (ν/сm–1): 1237 (СОС), 1558–1605 (Ar), 1675 and 1698 (С=О).
Biological activity
Preliminary plant growth regulation activity tests were performed on tobacco cell culture grown under strictly controlled conditions: temperature 26 °C, modified Schenk and Hildebrandt medium. The experiments were carried out at the compound concentrations of 5·10–6 mol/L; the results were collected in 120 h.
Effect of compounds 8 and 9 on the growth of vegetative organs of beans and oats. Belozernaya bean seeds and Orel oat seeds were sowed in 0.5 L paper cups filled with perlite. The plants were grown in racks equipped with fluorescent lamps for 10 days. Then, the plants were sprayed with solutions of the tested compounds (4 mg of the active compound per a cup). Two cups were used for each variant, and 3–4 bean plants or 10 oat plants were sowed in each cup. In two weeks, the total weight of oats and the total weight increase of beans per each variant were determined. Growth inhibitor MAH was used as a reference. The Results are given in Table 2.
Wheat drought resistance tests. Moskovskaya 35 spring wheat was grown in tap water for 4 days and in solutions of the tested compounds for 3 days. Ten-day wheat plants without roots and grains were slowly dried in a thermostat at 24 °C for a day; then they were stored in a wet chamber on a wet filter paper. In 7 days, the number of surviving plants was determined. The results are presented in Table 3.
Wheat frost resistance tests. Ilichevka winter wheat was germinated in a thermostat for 3 days and then grown in solutions of the tested compounds for 7 days. Ten-day wheat plants without roots, but with grains were tempered at 2 °С for 7 days, frozen at –5 °С for 24 h, and finally thawed out at room temperature. The plants were poured with distilled water for 2 hours, and the yields of electrolytes from the dead tissues were measured. The ratio of the yields of electrolytes in the frosted tissues to those in the dead tissues was considered as a measure of resistance of the plant tissues to negative temperatures—the lower this ratio, the higher the resistance.
Statistical analysis. Statistical processing of the results was performed using Microsoft Excel software. To assess the statistical significance of various datasets, the acceptable significance value was p = 0.05. 95% confidence intervals of the true averages are given in the Tables.
Conclusions
The compounds obtained by the reactions of N-alkoxycarbonylethylenediamines with aromatic isocyanates, thus, having ethylene-bridged carbamate and urea groups exhibited the high plant growth regulation activity along with the unique antistress properties, exceeding in the performance their structural analogs, such as kartolin-2. More importantly, the protective action of these substances against herbicides extends to phytotoxicants with both hormonal and antimetabolite action mechanisms.
Acknowledgements
The contribution of the Center for molecular composition studies of INEOS RAS is gratefully acknowledged.
This research was supported by the Russian Foundation for Basic Research (RFBR), project nos. 15-29-05785 ofi_m and 18-33-01128 mol_а.
References
- T. Fujiwara, D. O'Hagan, J. Fluorine Chem., 2014, 167, 16–29. DOI: 10.1016/j.jfluchem.2014.06.014
- L. Lombardo, G. Coppola, S. Zelasco, Trends Biotechnol., 2016, 34, 49–57. DOI: 10.1016/j.tibtech.2015.10.006
- Plant Propagation by Tissue Culture, 3rd ed., E. F. George, M. A. Hall, G.-J. De Klerk, Eds., Springer, 2008, vol. 1, ch. 5, pp. 175–204.
- E. V. V. Varejão, A. J. Demuner, L. C. A. Barbosa, R. W. Barreto, Crop Prot., 2013, 48, 41–50. DOI: 10.1016/j.cropro.2013.02.008
- E. Dumas, M. Giraudo, E. Goujon, M. Halma, E. Knhili, M. Stauffert, I. Batisson, P. Besse-Hoggan, J. Bohatier, P. Bouchard, H. Celle-Jeanton, M. Costa Gomes, F. Delbac, C. Forano, P. Goupil, N. Guix, P. Husson, G. Ledoigt, C. Mallet, C. Mousty, V. Prévot, C. Richard, S. Sarraute, J. Hazard. Mater., 2017, 325, 136–156. DOI: 10.1016/j.jhazmat.2016.11.059
- L. H. Ziska, Agric., Ecosyst. Environ., 2016, 231, 304–309. DOI: 10.1016/j.agee.2016.07.014
- R. A. Fletcher, A. Gilley, N. Sankhla, T. D. Davis, in: Horticultural Reviews, Wiley, J. Janick, Ed., 2000, vol. 24, ch. 3, pp. 55–138. DOI: 10.1002/9780470650776.ch3
- C. W. Coggins Jr, C. J. Lovatt, in: Citrus Production Manual, Richmond, ANR Communication Services, L. Ferguson, E. E. Grafton-Cardwell, Eds., 2014, pp. 215–226.
- K. Z. Gamburg O. N. Kulayeva, G. S. Muromtsev, L. D. Prusakova, D. I. Chkanikov, in: Plant Growth Regulators, G. S. Muromtsev, Ed., Moscow, Kolos, 1979, pp. 86–105 [in Russian].
- M. A. Ahanger, S. R. Tyagi, M. R. Wani, P. Ahmad, Adv. Agron., 2014, 25–55. DOI: 10.1007/978-1-4614-8591-9_2
- M. Asgher, M. I. R. Khan, N. A. Anjum, N. A. Khan, Protoplasma, 2015, 252, 399–413. DOI: 10.1007/s00709-014-0710-4
- L. Plíhalová, H. Vylíčilová, K. Doležal, L. Zahajská, M. Zatloukal, M. Strnad, New Biotechnol., 2016, 33, 614–624. DOI: 10.1016/j.nbt.2015.11.009
- A. S. Lukatkin, A. S. Semenova, A. A. Lukatkin, Agrokhimiya, 2016, 1, 73–95.
- M. Mazid, T. A. Khan, F. Mohammad, Biol. Med., 2011, 3, 232–249. DOI: 10.1007/s11694-015-9293-9
- A. Ricci, C. Bertoletti, Plant Biol., 2009, 11, 262–272. DOI: 10.1111/j.1438-8677.2008.00165.x
- D. W. S. Mok, M. C. Mok, Annu. Rev. Plant Physiol. Plant Mol. Biol., 2001, 52, 89–118. DOI: 10.1146/annurev.arplant.52.1.89
- E. Rolli, M. Incerti, F. Brunoni, P. Vicini, A. Ricci, Phytochemistry, 2012, 74, 159–165. DOI: 10.1016/j.phytochem.2011.10.012
- M. Kamínek, J. Plant Growth Regul., 2015, 34, 723–739. DOI: 10.1007/s00344-015-9543-4
- F. Brunoni, E. Rolli, L. Dramis, M. Incerti, D. Abarca, A. Pizarro, C. Diaz-Sala, A. Ricci, Plant Cell, Tissue Organ Cult., 2014, 118, 111–124. DOI: 10.1007/s11240-014-0466-8
- G. M. Stoilkova, P. Yonova, K. Ananieva, Plant Growth Regul., 2014, 72, 303–312. DOI: 10.1007/s10725-013-9861-0
- P. Yonova, Gen. Appl. Plant Physiol., 2010, 36, 124–147.
- Y. A. Baskakov, Agrokhimiya, 1988, 4, 103–105.
- K. A. Kochetkov, L. V. Kovalenko, A. V. Kalistratova, M. S. Oshchepkov, M. M. Vorob'ev, P. S. Protopopova, RF Patent 2632466, 2017.
- K. Naoki, H. Hiroshi, N. Yutaka, S. Hideyuki, Jpn. Patent 05295017, 1993.





