Issue 2
|
|
INEOS OPEN, 2018, 1(2), 94–97 Journal of Nesmeyanov Institute of Organoelement Compounds DOI: 10.32931/io1808a |
|
Modified Polyphenylquinoxalines
for Proton Conducting Membranes
a Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, Moscow, 119991 Russia
b Department of Materials Science and Engineering, National Cheng-Kung University, 1 University Road, Tainan City, 70101 Taiwan (R. O. C.)
Corresponding author: E. G. Bulycheva, e-mail: bulychev@ineos.ac.ru
Received 22 May 2018; accepted 10 September 2018
Abstract

The hydrogen atoms of the sulfo groups of sulfonated polyphenylquinoxalines are substituted for the alkali metal ions through polymer analogous reactions. The thermal, strength and proton conducting properties of the resulting ionomers and their nanocomposites with montmorillonite are studied before and after doping in orthophosphoric acid.
Key words: sulfonated polyphenylquinoxalines, ionomers, doping, montmorillonite, proton conducting membranes.
Introduction
Proton conducting membranes play a key role in fuel cells, since they serve as a proton conducting medium and as a barrier that allows avoiding direct contact between a fuel and an oxidizing agent [1, 2].
A reduction of the detrimental effect of СO contained in fuel [3] as well as those of other impurities is an urgent task, the solution of which will enable the use of cheaper (impure) hydrogen derived, for example, from reforming of associated petroleum gas and light hydrocarbons.
One of the methods for addressing the problem of catalyst poisoning is to rise operational temperatures of proton conducting membranes over 100 °С [4].
Owing to their high thermal stability and good mechanical properties, polyphenylquinoxalines (PPQ) seem to be promising materials for high-temperature (>100 °С) fuel cells. However, it was noted [5] that doping of PPQ with orthophosphoric acid drastically deteriorates the stability of polymer samples, especially at elevated temperatures. This is likely to result from the effect of plasticization by sorbed phosphoric acid and associated water. The doped films from PPQ convert to soft gels which cannot be used as membranes due to their mechanical properties.
Sulfonated polyphenylqinoxalines (SPPQ) that result from a polymer analogous transformation of PPQ upon sulfonation with a mixture of sulfuric acid and oleum [6] can be considered as potential effective heat-resistant polymer electrolytes and as a basis for production of promising heat-resistant aromatic ionomers, assigned for application in different fields.
Earlier some of us showed [7] that an increase in the sulfonation degree of SPPQ from 1.7% to 8.0% of the sulfur content improves the proton conductivity at room temperature from 4.9·10–6 to 2.4·10–3 S·cm–1, which can be explained by the appearance of a continuous hydrogen bond network.
Subsequent doping of SPPQ with orthophosphoric acid led to the fact that the films dissolved in it or lost their sizes or shapes and could not be explored. As a result, we failed to reach the desired improvement of proton conductivity.
Therefore, it seemed reasonable to accomplish a polymer analogous transformation of SPPQ through the substitution of the hydrogen ions in the sulfo groups for the alkali metal ions in order to obtain the corresponding ionomeric SPPQ structures which are expected to exhibit the improved stability.
Results and discussion
The main principle of the suggested approach consists in the fact that blocking of the sulfo groups in the resulting physical cross-links must hamper the progress of thermochemical destruction, which affords a new approach to stabilization of polymer electrolytes.
The reaction was carried out under heterogeneous conditions on SPPQ films bearing 2 to 9% of sulfur according to the published procedure (Scheme 1) [8].

Scheme 1. Ionomeric structures based on PPQ.
The complete substitution of the hydrogen atoms in the sulfo groups was confirmed by the amount of the metal included in the polymer, which was defined by means of nondestructive X-ray fluorescence.
Table 1 lists the strength characteristics of the films of the initial ionomer based on SPPQ bearing 3.5% of sulfur before and after doping (doping conditions: duration 1 h, temperature 70 °C, 14 M orthophosphoric acid).
Table 1. Mechanical properties of SPPQ-Na ionomeric membranes before and after doping
|
Entry |
Sample |
Tensile |
Relative elongation [%] |
Elasticity |
|
1 |
SPPQ-Na |
41.81 ± 0.92 |
9.67 ± 1.02 |
0.72 ± 0.04 |
|
2 |
SPPQ-Na |
23.46 ± 1.62 |
9.02±0.14 |
0.67 ± 0.04 |
As can be seen from the data presented, the strength of this film after doping somewhat reduces but still remains high enough to enable the investigation of its proton conducting properties at 180 °С.
The greatest changes in the physicochemical properties could be expected upon introduction of an anisometric nanosized filler into the polymer. A considerable degree of anisometry is characteristic of the particles of layered aluminosilicates, in particular montmorillonite (natural aluminosilicate from a subclass of layered materials with variable chemical compositions: (ОН)4Si8(Al3.34Mg0.67)O20Me0.67) (Nanoclay, Aldrich). It should be noted that the strength characteristics upon introduction of organic and inorganic molecules into the polymer matrix were studied earlier by the example of polybenzimidazole (PBI) [9, 10].
To provide an optimal balance of the hydrophilic-hydrophobic properties of the aluminosilicate surface, it was modified by the treatment with an ammonium salt of dodecylamine, which gave rise to the corresponding organic clay (MMT). The introduction of organic cations led to an increase in the interplanar spacing of the montmorillonite lattice, which, in turn, improved the clay dispersion over the polymer matrix. The production of SPPQ-Na nanocompositions with MMT according to the previous studies with other polymers (predominantly PBI) [11–13] must have positively affected the mechanical properties of new ionomeric SPPQ-Na membranes.
SPPQ-Na + MMT ionomeric membranes were produced according to the following procedure.
SPPQ (0.75 g, 3.5% of sulfur) and MMT (0.0395 g) were dissolved in 4 mL of DMAA (the content of a solid phase was about 16%) for 24 h. The resulting solution was poured onto a glass and dried under vacuum at 80 °C for 12 h. The thickness of the film obtained was about 50 μm. It was placed in 10% aq. NaOH and left for 7 days at 20 °С. Then the film was rinsed with water till neutral pH and dried at 100 °C till the constant mass. The membranes with 3% and 7% of MMT were prepared analogously.
Exfoliation of the filler in SPPQ-Na matrix was confirmed by transmission electron microscopy (Figs. 1a,b). The dark lines on the transmission electron micrographs correspond to the nanolayers of MMT distributed over the polymer matrix. At the content of MMT of 7%, there was observed the aggregation of silicate layers (Fig. 1c).
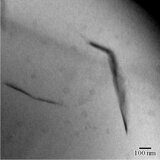
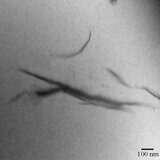
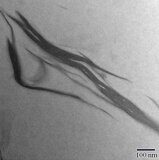
Figure 1. TEM micrographs of SPPQ-Na with different contents of MMT: 3% (a), 5% (b), and 7% (c).
Investigation of the thermal characteristics of ionomeric SPPQ-Na showed that the temperatures of decomposition beginning (till 10%) according to the TGA data almost do not depend on the amount of MMT introduced (Fig. 2). Above 450 °С the masses of the composite residues were higher than that of SPPQ-Na and also almost did not depend on the content of MMT (Fig. 2).
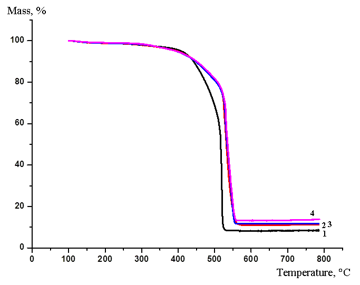
Figure 2. TGA curves for ionomeric SPPQ-Na (curve 1) and its nanocomposites with MMT (MMT content:
3% (curve 2), 5% (curve 3), and 7% (curve 4)) registered in air.
Analogous dependences were described earlier for the modified samples of PBI [10]. Presumably, the particles of the disperse clay protect the polymer chains from the impact of oxidizing radicals at high temperatures, which leads to some improvement of the oxidative stability of the resulting nanocomposites compared to neat SPPQ-Na.
Figure 3 depicts the dependences of the glass-transition points on the content of MMT in a SPPQ-Na ionomer sample.
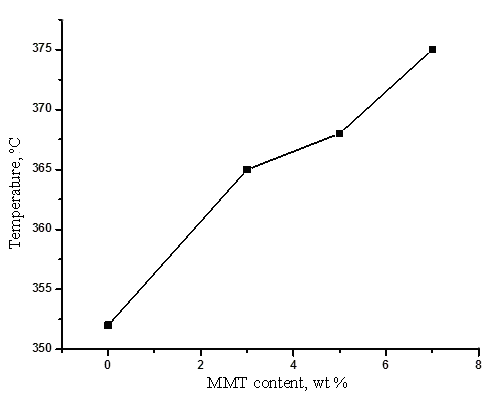
Figure 3. Dependence of the glass-transition point of SPPQ-Na ionomer on MMT content.
The data presented in Fig. 3 indicate that an increase in the content of MMT to 7% leads to a rise in the sample glass-transition point by 22 °С.
Table 2 shows the results of comparative investigations on the mechanical properties of SPPQ ionomeric films after their modification with MMT and doping in H3PO4.
Table 2. Mechanical properties of the initial SPPQ-Na ionomeric
membrane and its nanocomposites with MMT before and after doping
|
Entry |
Sample |
Tensile strength |
Relative elongation |
Elasticity modulus |
|
1 |
SPPQ-Na |
41.81 ± 0.92 |
9.67 ± 1.02 |
0.72 ± 0.04 |
|
2 |
SPPQ-Na + |
44.92 ± 0.71 |
7.80 ± 0.67 |
0.83 ± 0.08 |
|
3 |
SPPQ-Na + |
46.07 ± 0.72 |
8.44 ± 1.19 |
0.82 ± 0.03 |
|
4 |
SPPQ-Na + |
43.81 ± 1.30 |
8.00 ± 1.58 |
0.76 ± 0.08 |
|
5 |
SPPQa |
23.46 ± 1.62 |
9.02 ± 0.14 |
0.67 ± 0.04 |
|
6 |
SPPQ-Na + |
31.48 ± 1.87 |
6.70 ± 0.84 |
0.57 ± 0.03 |
|
7 |
SPPQ-Na + |
36.60 ± 2.46 |
6.65 ± 0.27 |
0.78 ± 0.03 |
|
8 |
SPPQ-Na + |
32.13 ± 2.11 |
7.96 ± 1.35 |
0.58 ± 0.05 |
Filling of SPPQ-Na with MMT nanoparticles at their low content (3–5%) leads to an increase in the tensile strength and tensile modulus, which is likely to be caused by the formation of a great amount of anisotropic nanoparticles that serve as strengthening elements in the polymer matrix. Thus, at the montmorillonite content of 3 wt %, the elasticity modulus of the material grows by 17%, and its tensile strength increases by 7%. At the same time, the relative elongation reduces insignificantly.
When the amount of MMT reaches 7%, the mechanical characteristics of the nanocomposite film begin to deteriorate. This behavior can be associated with the aggregation of silicate layers in the composite film, which was confirmed by the electron microscopy data (Fig. 1с).
Comparison of the strength characteristics of the doped samples with those of the analogous membranes before doping (Table 2) revealed that, although the elasticity modulus and strength of the films reduce after doping, this process is less pronounced in the samples modified with MMT than in the samples lacking MMT. This evidences that the application of MMT in SPPQ-Na ionomeric membranes efficiently reduces the plasticizing effect of orthophosphoric acid and affords doped SPPQ-Na membranes with reasonably high mechanical parameters.
Good strength characteristics of SPPQ-Na membranes and the composites with MMT allowed us to study their proton conductivities at 180 °C. The data are presented in Table 3.
Table 3. Doping degrees and proton conductivities at 180 °of SPPQ-Na ionomeric
membrane and its composites with MMT
|
Entry |
Sample |
Doping degree [%] |
Proton conductivity at 180 °C [S·cm–1] |
|
1 |
SPPQ-Na |
174 |
0.0297 |
|
2 |
SPPQ-Na + 3% MMT |
161 |
0.0219 |
|
3 |
SPPQ-Na + 5% MMT |
156 |
0.0181 |
|
4 |
SPPQ-Na + 7% MMT |
142 |
0.0179 |
The results obtained are likely to be explained by the fact that MMT dispersed over the polymer matrix can decelerate the mobility of protons in membranes. It should be noted that the analogous dependences of proton conductivities of the membranes after introduction of MMT were established earlier for PBI at 25 and 70 оC [14].
Conclusions
The results obtained evidence that the introduction of MMT into SPPQ ionomeric membranes increases their thermooxidative stabilities and glass-transition points and improves the mechanical properties of doped nanocomposite membranes, which opens the way to systems that show great potential as high-temperature proton conducting membranes.
Acknowledgements
This work was supported by the Russian Foundation for Basic Research, project no. 16-53-52032 MNT_a, and the Ministry of Science and Technology of Taiwan (R. O. C.), project no. MOST 105-2923-E-006-003-MY3.
References
- J. Larminie, A. Dicks, Fuel Cell Systems Explained, Wiley, Chichester, 2000.
- M. Rikukawa, K. Sanui, Prog. Polym. Sci., 2000, 25, 1463–1502. DOI: 10.1016/S0079-6700(00)00032-0
- Q. Li, R. He, J.-A. Gao, J. O. Jensen, N. J. Bjerrum, J. Electrochem. Soc., 2003, 150, А1599–А1605. DOI: 10.1149/1.1619984
- S.-W. Chuang, S. L.-C. Hsu, Y.-H. Liu, J. Membr. Sci., 2007, 305, 353–363. DOI: 10.1016/j.memsci.2007.08.033
- M. A. Hickner, H. Ghassemi, Y. S. Kim, B. R. Einsla, J. E. McGrath, Chem. Rev., 2004, 104, 4587–4612. DOI: 10.1021/cr020711a
- A. L. Rusanov, N. M. Belomoina, E. G. Bulycheva, N. A. Yanul, D. Yu. Likhatchev, Y. A. Dobrovolskii, C. Iojоiu, J.-Y. Sanchez, V. Yu. Voytekunas, M. J. M. Abadie, High Perform. Polym., 2008, 20, 627–641. DOI: 10.1177/0954008307082446
- A. L. Rusanov, Yu. A. Dobrovolsky, Е. V. Gerasimova, N. M. Belomoina, Е. G. Bulycheva, in Unique Properties of Composites and Polymers: Pure and Applied Science Today and Tomorrow, Y. N. Bubnov, V. A. Vasnev, A. A. Askadskii, G. E. Zaikov, Eds., Nova Sci. Publ., New York, 2012, Vol. 2, 83–89.
- V. G. Vasil'ev, M. I. Buzin, G. G. Nikiforova, N. M. Belomoina, E. G. Bulycheva, V. S. Papkov, Dokl. Phys. Chem., 2014, 458, 149–152. DOI: 10.1134/S0012501614100029
- F. Ublekov, H. Penchev, V. Georgiev, I. Radev, V. Sinigersky, Mater. Lett., 2014, 135, 5–7. DOI: 10.1016/j.matlet.2014.07.128
- S. Singha, T. Jana, Polymer, 2016, 98, 20–31. DOI: 10.1016/j.polymer.2016.06.007
- S.-W. Chuang, S. L.-C. Hsu, C.-L. Hsu, J. Power Sources, 2007, 168, 172–177. DOI: 10.1016/j.jpowsour.2007.03.021
- J. Zhang, B.-K. Zhu, H.-J. Chu, Y.-Y. Xu, J. Appl. Polym. Sci., 2005, 97, 20–24. DOI: 10.1002/app.21721
- M. Aparicio, Y. Castro, A. Duran, Solid State Ionics, 2005, 176, 333–340. DOI: 10.1016/j.ssi.2004.07.021
- M. M. Hasani-Sadrabadi, N. M. Dorri, S. R. Ghaffarian, E. Dashtimoghadam, K. Sarikhani, F. S. Majedi, J. Appl. Polym. Sci., 2010, 117, 1227–1233. DOI: 10.1002/app.31974





