Issue 1
|
|
INEOS OPEN, 2018, 1(1), 64–70 Journal of Nesmeyanov Institute of Organoelement Compounds DOI: 10.32931/io1805a |
|
and Properties of Glass-Reinforced Plastics
O. N. Zabegaeva,*a D. A. Sapozhnikov,a B. A. Bayminov,a
S. A. Zinov'eva,b T. N. Prudskova,b and Ya. S. Vygodskiia
a Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, Moscow, 119991 Russia
b Petrov Institute of Plastics, Perovskii proezd 35, Moscow, 111024 Russia
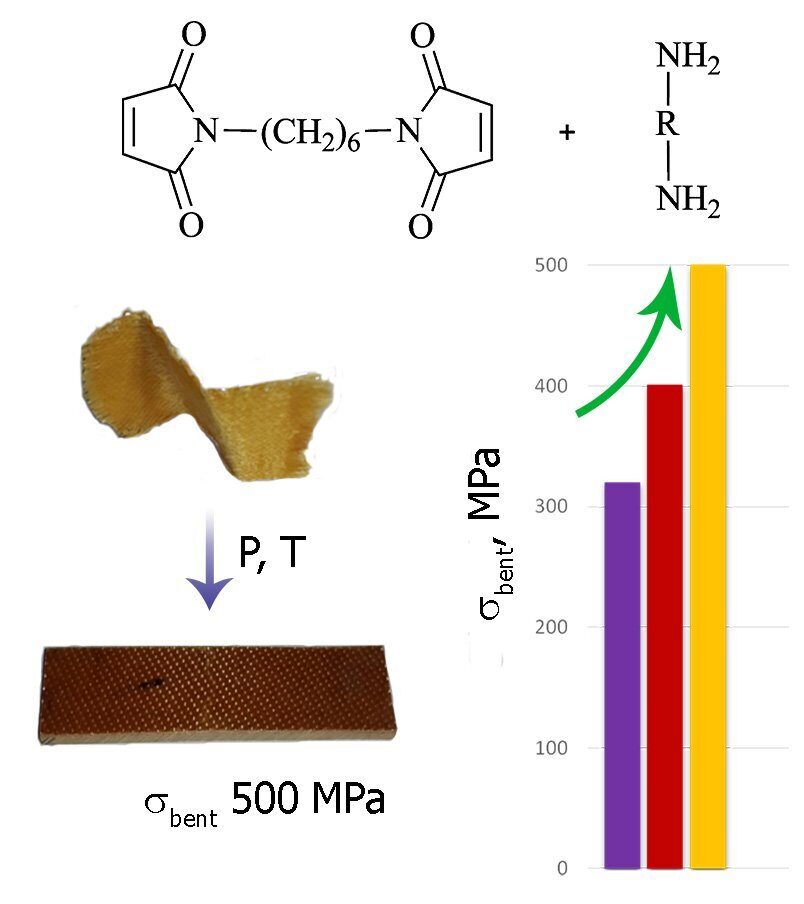
A series of fiberglass prepregs are obtained by dry impregnation using N,N'-hexamethylene bis(maleimide) and its mixtures with diamines as binding agents. The optimal modes for pressing of glass-reinforced plastics are determined by a rheokinetic technique. It is shown that the nature of a diamine strongly affects both the curing process of a polymer matrix and the properties of copolymers. It is found that the copolymerization of hexamethylene bis(maleimide) with aniline fluorene or a siloxane-containing diamine leads to improvement of stress–strain characteristics of the resulting glass-reinforced plastics.
Key words: bis(maleimide); polyimide; glass-reinforced plastic.
Introduction
Nowadays one of the most promising and popular types of structural materials are polymer composite systems which uniquely combine the properties of their components. The main technical requirements to these materials often appear to be crucial for specific tasks in different fields such as electronics, automobile and aerospace technologies. They include, in particular, high strength and thermal stability.
A fundamental problem in the creation of polymer composite materials is the choice of a polymer matrix that must provide the highest characteristics of a composite and comply with the specified technological and operational requirements.
Among the high-technology, heat and thermally resistant polymers, of utmost theoretical and practical importance are polyimides, which can stand prolonged heating at the temperatures over 300 °C without significant loss of strength characteristics [1]. However, the production of these polymers is accompanied by the release of water and other low-molecular products, which along with the necessity to remove polar solvents, the high viscosity of melts, and the low solubility reduce the application scope of these matrices.
Over recent years particular attention has been drawn to the production of thermosetting polyimides based on the monomers that contain reactive maleimide groups [2–4]. On the one hand, interest to these polyimides stems from their cross-linked structures, which provide the high strength properties; on the other hand, the formation of a three-dimensional polymer network proceeds without the release of any by-products which complicate the production of linear aromatic polyimides and deteriorate their properties. The advantages of bis(maleimide) resins comprise low coefficients of thermal expansion, fire and water resistance, availability of the starting reagents, and facile synthesis and processing [2–6]. The main disadvantage of bis(maleimide) resins is their enhanced brittleness which is caused by the high density of cross-linking of the polymer matrix [7–9]. This disadvantage can be removed by an increase in the polymer molecular mass [10] or modification of polyimides with elastomers or thermoplastics, which are able to strengthen the cured compositions [2, 3, 11–13]. The thermoplastic modifiers are heat-resistant polymers such as poly(ether sulfones) [14–17], poly(ether imides) [18], poly(ether ketones) [19], and so on. Upon curing, thermoplastic and polyimide form interpenetrating networks. At first sight, an idea to combine the thermoplastic polymers featuring high fracture viscosity with brittle bis(maleimide) resins in a single composition seems to be promising. However, this leads to great technological difficulties which are caused by the low solubility of thermoplastics and thermodynamic incompatibility of the mixture components, which can result in phase separations and, as a consequence, to deterioration of strength properties of the composites [14, 16, 17, 19].
A combination of bis(maleimide) resins with other high-technology thermosetting resins can ensure improvement of strength characteristics of the resulting composites. The choice of comonomers, their stoichiometry, and curing conditions plays a key role. The modification of bis(maleimides) with epoxides [8, 20, 21], cyanates [9, 22, 23], propargyl ethers [20], and benzoxazines [24–26] was reported. Unlike the above-mentioned modification of bis(maleimide) resins with thermoplastics, in this case the formation of a network polyimide occurs owing to the parallel or sequential reactions of linear migration copolymerization (the Michael addition) and three-dimensional polymerization of bis(maleimides) by the double C=C bonds. If the migration copolymerization prevails over the homopolymerization of a bis(maleimide), the resulting copolymer will feature a more loose network than the polymer obtained under conditions of homopolymerization. Naturally, the conditions of copolymerization (the ratio of reagents, the temperature mode, the duration of curing, etc.) affect the physicochemical properties of copolymers, even when they are obtained from one and the same starting comonomers. The authors of the mentioned works noted an increase in the strength of the composite materials by 20–30%; at the same time, the thermal stability and heat resistance considerably reduce compared to those of the unmodified bis(maleimide). In some cases, in contrast, a serious deterioration in the strengths of the resulting items was reported that can be associated with the low adhesion of the system components which leads to phase separation [25, 27, 28].
The present work deals with investigation of the properties of composite materials based on three-dimensional copolymers of N,N'-hexamethylene bis(maleimide) (HMBMI) and different diamines. The choice of these compounds was dictated by the fact that bis(maleimides) of aliphatic diamines, in particular, HMBMI feature relatively low melting points, which allows one to form a three-dimensional polymer network in melt under relatively mild temperature conditions. On the other hand, the high solubility of linear high-molecular polyimides based on card, siloxane-containing and partially aliphatic diamines allowed us to expect good compatibility of these diamines with bis(maleimides), which might favorably affect the strength properties of the resulting polymers. It should be noted that the operational properties of this type of composites are described mainly for the prepregs molded from solutions, which is complicated by the high viscosity of the solutions and necessity to thoroughly remove a solvent [29–31]. In our opinion, a solvent-free dry method for creation of composite prepregs is simpler and less energy-consuming. Fiberglass was chosen as a reinforcing agent owing to its high mechanical strength along with the flexibility, availability, and simplicity of handling.
Results and discussion
As it was mentioned in Introduction, the homopolymerization of a bis(maleimide), which leads to the formation of a three-dimensional polymer, is accompanied by the migration copolymerization of the bis(maleimide) with a diamine (Scheme 1).
The probability of predominance of one or another reaction depends mainly on the temperature–time conditions of curing and on the type of a diamine in use [32]. Since a literature survey did not reveal the information on rheological characteristics of melts of this type of monomers, it seemed reasonable to conduct such an investigation. We studied the effect of the factors such as temperature, reaction duration, and diamine additives on the process of HMBMI curing. Bis(maleimide):diamine molar ratios were as follows: 1.0:0.5 in the case of aniline fluorene (AFL) and diamine A and 1.0:0.2 in the case of the siloxane-containing diamine, 1,3-bis(3-aminopropyl)-1,1,3,3-tetramethyldisiloxane (ADS).
Heating of HMBMI with the rate of 2 deg/min revealed a drastic growth in the melt viscosity at 180 °C (Fig. 1). The monomer conversion and the yield of a cross-linked product at the gel point comprised 99.5 and 94.0 %, respectively (Table 1). At the same time, upon heating of HMBMI in the presence of diamines, a drastic growth in the melt viscosities was detected at 10–20 deg higher, except for a mixture with the siloxane-containing diamine. The earlier observed growth of the viscosity of HMBMI:ADS system is likely to be caused by the high reactivity of this diamine due to its higher basicity compared to the aromatic amines. The gel yield in this case reached 92% at almost complete conversion of the monomers (99.5%). The reaction of 2,2-bis[4-(4-aminophenoxy)phenyl]propane (diamine A) with HMBMI afforded a three-dimensional polyimide with the lowest conversion (70%) among the diamines explored at the content of the gel fraction of 80%.
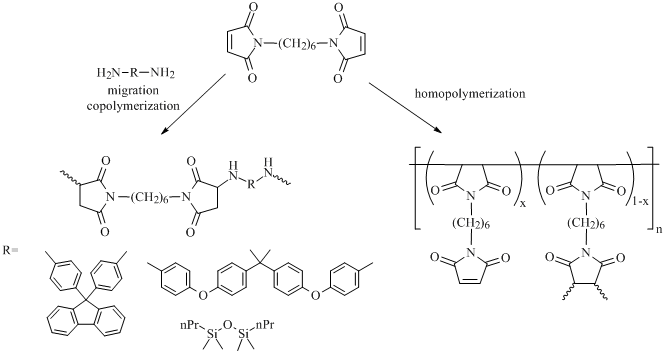
Scheme 1. Possible reactions between N,N'-hexamethylene bis(maleimide) and diamines.
The work was concerned with the following diamines: 9,9-bis(4-aminophenyl)fluorene (aniline fluorene, AFL),
2,2-bis[4-(4-aminophenoxy)phenyl]propane (diamine A), and 1,3-bis(3-aminopropyl)-1,1,3,3-tetramethyldisiloxane (ADS).
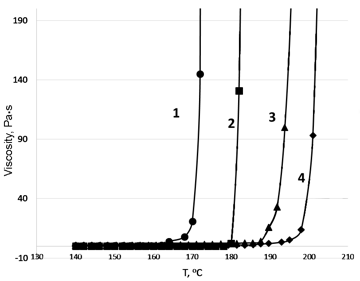
Figure 1. Rheokinetic curves of curing of HMBMI (2) and its mixtures with the siloxane diamine (1), aniline fluorene (3), and diamine A (4) registered in a dynamic mode of heating from 140 to 210 °C.
Table 1. Temperature–time parameters of resin curing.
|
Mixture composition |
Dynamic mode (140–250 °С)a |
Static mode (150 °С)b |
||
|
Ѡ,c % |
G. fr.,d % |
Ѡ,c % |
G. fr.,d % |
|
|
HMBMI |
99.5 |
94 |
23 |
58 |
|
HMBMI:AFL |
99.5 |
90 |
20 |
53 |
|
HMBMI:ADS |
99.5 |
92 |
51 |
90 |
|
HMBMI:diamine A |
70.0 |
80 |
11 |
– |
b seasoning of the samples for 3.5 h at 150 °C;
c Ѡ – conversion of the monomers after extraction with acetone;
d G. fr. – gel fraction of the (co)polymers after extraction with NMP
To give further insight into the observed processes, the rheological tests were carried out under conditions of static heating. Upon heating of HMBMI at 150 °C in the absence of diamines (Fig. 2A), the gel formation started in 55 min and a cross-linked polymer was formed in 4 min; therewith, the yield of a fraction insoluble in NMP at the gel point composed 58%. The results of investigation on the behavior of HMBMI mixture melts at 150 °C showed that ADS started to react with the bis(maleimide) in 10–13 min after heating beginning (Fig. 2B). This reaction proceeded intensively for the following 6–10 min. Presumably, this period was characterized mainly by the migration polymerization, whereas the homopolymerization of the bis(maleimide) almost did not take place. Further heating at 150 °C led to a drastic growth in the melt viscosity and afforded the product in 51% yield with the content of the NMP-insoluble fraction of 90%.
A growth in the viscosity of the mixtures of HMBMI with diamine A or aniline fluorene started considerably later, and a time interval that characterizes the polymerization was expressed in a greater extent than in the case of the mixture with ADS (Figs. 2C, 2D). Seasoning of these systems for more than 3 h at 150 °C gave rise to the copolymers with relatively low conversions equal to 10–20%.
Based on the resulting data, it can be assumed that the diamines serve as inhibitors of bis(maleimide) homopolymerization. At the beginning of the process, the migration copolymerization is likely to prevail over the polymerization by the double C=C bonds.
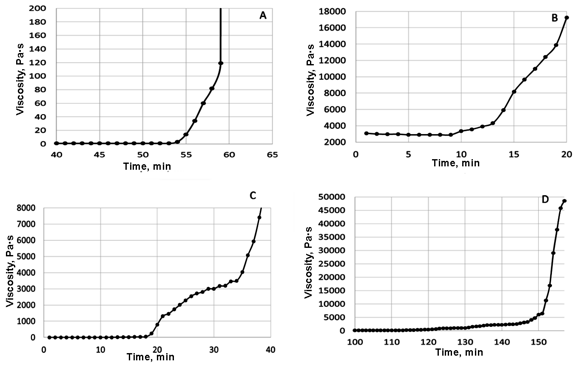
Figure 2. Rheokinetic curves of curing of HMBMI (A) and its mixture with the siloxane diamine (B), aniline fluorene (C), and diamine A (D) registered in a static mode of heating at 150 °C.
The processes occurring upon heating of the systems under consideration were studied in detail using differential scanning calorimetry (DSC) (Fig. 3). The curve obtained for neat HMBMI shows an endothermic peak with a maximum at 140 °C, corresponding to melting of the bis(maleimide), which then converts to an exothermic peak in the range of 240 °C, caused by the homopolymerization of the latter. The analysis of the DSC curves for the mixtures revealed a smooth change in the heat capacity almost immediately after a peak in the range of 140 °C, which is responsible for the monomer melting. This smooth change converts to a broad exothermic peak which corresponds to the processes of homo- and copolymerization.
Hence, the results of the rheological and DSC studies allowed us to define the conditions for impregnation of a fiberglass with the binding agents based on HMBMI and to develop an optimal mode for the following curing of the molded prepregs.
A series of prepregs were prepared by dry application of the monomer mixtures. A fraction of the monomers calculated per the prepreg composed 40 wt %. The fiberglass impregnation was carried out at 145–150 °C; under these conditions, the diamines readily dissolved in HMBMI, resulting in homogeneous low-viscous melts, and the polymerization processes were substantially retarded. The prepregs molded from HMBMI and diamine A or AFL were more flexible and more prone to mechanical impact than the fiberglass containing only HMBMI. Twisting and bending of these prepregs were not accompanied by embrittlement or shattering of a part of the binding agent in contrast to the materials with HMBMI (Fig. 4). The use of ADS allowed us to obtain a soft and even more flexible prepreg that can be twisted in any forms which is especially important for production of items of intricate shapes.
Based on the results of investigation of the rheological properties and propensity of the low-viscosity binder to pressing from the prepreg, we developed an optimal mode for the direct hot pressing. The pressing process consists in a simultaneous impact of pressure and temperature on the material. The first step of the pressing was carried out at 160 °C under contact pressure, which provided firm adherence of the heated plates of the press to the molded block of the prepreg without further pressing of the binder. At these temperatures, when the viscosity of the molten binder was rather low and the reactions of homo- and copolymerization almost did not proceed, the fiberglass sheets were impregnated additionally with the melt which stuck them with each other. Subsequently, in order to increase the viscosity of the binding agent due to intensive polymerization processes, the temperature was elevated to 195–200 °C, after which the pressure was enhanced to 5.5 ± 0.5 kg/cm2. Under these conditions, the resin was finally cured and stuck the fiberglass layers with each other, resulting in a compact monolithic sheet. For the complete curing, the reaction mixture was heated to 225 °C and kept at this temperature for 3 h. The resulting glass-reinforced plastics were uniform, light- to dark-brown, and strong, without any visible defects (Fig. 5).
The effect of chemical structures of the resulting glass-reinforced plastics on their thermal stabilities was studied. According to the results of dynamic thermogravimetric analysis in air, the temperatures of the mass loss beginning for almost all the network polymers derived lie in the range of 400 °C and only slightly differ from each other. The highest temperature of 5% mass loss was detected for the homopolymer, which is likely to be connected with the high cross-linking density of the polymer chains. The introduction of diamines into the reaction reduced the thermal stability of the copolymers by 10–30 °C (Table 2). The use of diamines in the reaction is likely to lead to the formation of linear aminoimide fragments (Scheme 1) which violates the regularity and density of the polymer network; consequently, these copolymers featured lower thermal stabilities than the homopolymer.
The thermomechanical analysis of the resulting glass-reinforced plastics did not reveal any essential effect of the chemical structures of three-dimensional copolymers on their softening points. All the polymers (both homo- and copolymers) possess high enough heat resistances and do not deform up to 400 °C, i.e., up to the temperature of beginning of thermal oxidation.
Scheme 2. Branching of the polymer chain upon copolymerization of bis(maleimide) with diamine.
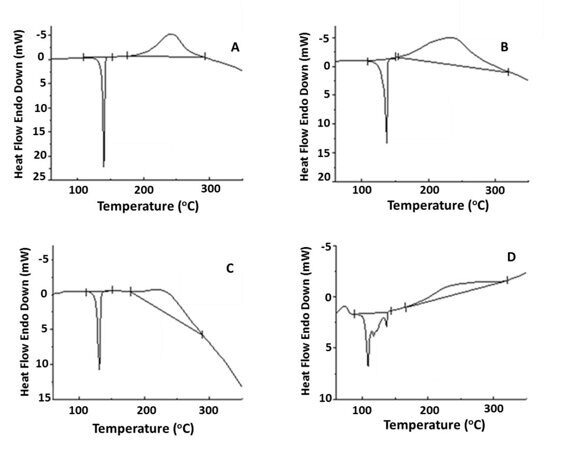
Figure 3. DSC curves for neat HMBMI (A) and its mixture with the siloxane diamine (B),
aniline fluorene (C), and diamine A (D).
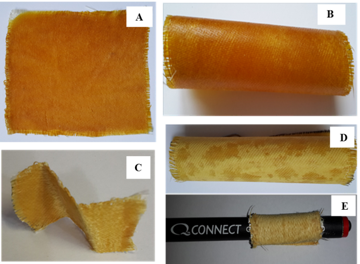
Figure 4. Photographs of the prepregs based on HMBMI (A) and its mixtures with AFL (B),
diamine A (C), and ADS (D, E).
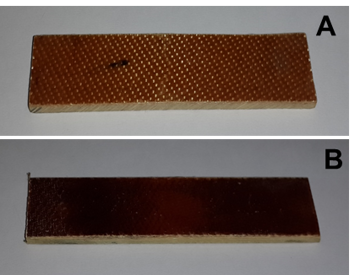
Figure 5. Photographs of the glass-reinforced plastics based on HMBMI (A) and its copolymer with AFL (B).
Table 2. Properties of the glass-reinforced plastics based on different binders.
|
Binder |
Т5%, °С |
σbend, MPa |
Еbend, MPa |
|
HMBMI |
410 |
330 ± 16 |
25400 ± 1400 |
|
HMBMI:AFL |
405 |
500 ± 14 |
24000 ± 1900 |
|
HMBMI:ADS |
395 |
410 ± 30 |
20500 ± 300 |
|
HMBMI:diamine A |
380 |
260 ± 37 |
22700 ± 800 |
It was shown that the strength properties of the molded glass-reinforced plastics strongly depend on the nature of the polymer matrix. Thus, the dilution of the polyHMBMI network with aniline fluorene fragments led to an increase in the bending strength by a factor of 1.5, which reached 500 MPa at almost unchanged elasticity modulus (Table 2). The ADS-modified bis(maleimide) matrix was slightly inferior in the strength properties than the glass-reinforced plastic with AFL but excelled the composite based on the homopolymer. It is interesting to note that in the latter case the elasticity modulus was essentially lower than for two former systems. The flexible siloxane chains are likely to serve as plasticizers, thus, reducing the brittleness of a plastic. The lowest strength among the glass-reinforced plastics explored was detected for that based on HMBMI and diamine A. This effect is likely to be caused by incomplete conversion of the monomers under conditions of pressing due to the low reactivity of the diamine in use.
Experimental
Starting compounds
1,6-Hexamethylenediamine was distilled over KOH. 1,3-Bis(3-aminopropyl)-1,1,3,3-tetramethyldisiloxane was purchased from Dow Corning Toray and purified by vacuum distillation (bp = 133–145 °C, 10–15 mmHg). 9,9-Bis(4-aminophenyl)fluorene was sublimed at 20 Pa. 2,2-Bis[4-(4-aminophenoxy)phenyl]propane was purified by crystallization from 15–20 wt % solution in a water–isopropanol mixture (20:80 vol %) with addition of several drops of hydrazine hydrate. The melting and boiling points of the monomers corresponded to the reported ones [33]. Maleic anhydride (99%, Acros Organics), sodium bicarbonate (Spektrkhim), and glacial acetic acid (reagent grade, Spektrkhim) were used as received without further purification.
N,N'-Hexamethylene bis(maleimide) was obtained by the reaction of maleic anhydride and 1,6-hexamethylenediamine in acetic acid according to the published procedure [34]. The identity of the resulting bis(maleimide) was confirmed based on the data of elemental analysis, IR and 1H NMR spectroscopy. Anal. Calcd. for C14H16N2O4: C, 61.0; H, 5.8; N 10.0. Found: C, 59.8; H, 5.8; N 9.8%. The IR spectrum of the bis(maleimide) showed the absorption bands at 1375 and 1686 cm–1, which correspond to the characteristic vibrations of the C–N and C=O bonds in the imide ring, and the absorption band at 3089 cm–1, which is associated with the stretching vibrations of the C–H bonds at the C=C bonds. The 1H NMR spectrum of HMBMI (400.15 MHz, DMSO-d6) showed the signals corresponding to the protons of the HC=CH moieties (δH = 6.70 ppm, d, 4 H) and the hexamethylene fragment (δH = 3.38 ppm, t, 4 H, CH2N; δH = 1.46 ppm, m, 4 H, CH2CH2N; δH = 1.20 ppm, m, 4 H, CH2CH2CH2N). The integral intensities of these signals were consistent with the structure of HMBMI.
The prepregs (10 × 10 cm) were obtained by impregnation of T-10-14 fiberglass with the melt of N,N'-hexamethylene bis(maleimide) or its mixtures with the diamines. Bis(maleimide):diamine molar ratios were 1.0:0.5 in the case of aniline fluorene and diamine A and 1.0:0.2 in the case of the siloxane-containing diamine. The fraction of the bis(maleimide) (or its mixture) composed 40 wt % from that of the prepreg.
Preparation of the glass-reinforced plastics
A sample of the glass-reinforced plastic with the thickness of ~2 mm was obtained by the high-temperature pressing of the molded prepregs on a hydraulic press of PH-M 63 T type (Poland). The pressing mode was as follows: preliminary heating to 160 °C under contact pressure, heating to 200 °C and increase of the pressure to 5.5 ± 0.5 kgf/cm2, and further seasoning at 225 °C for 3 h.
Investigation methods
The IR spectra were registered on a Perkin Elmer Spectrum One FTIR spectrometer (USA) equipped with a UATR accessory (Di/ZnSe crystal, single reflection). The NMR spectra were recorded on a Varian VNMR-400 spectrometer (USA) in DMSO-d6 using residual proton signals of the deuterated solvent as an internal standard (1H, 13C). The dynamic viscosity of the melt was determined on a TA Instruments AR200ex rheometer at the heating rate of 2 deg/min and the shear rate of 0.1 s–2. The conversion of the monomers was evaluated based on the results of extraction of the ground polymers with acetone for 20 h. The gel fraction was estimated by the content of the polymer preliminary extracted with acetone which was insoluble in N-methyl-2-pyrrolidone. The thermal stabilities of the polymers were determined by TGA on a Perkin Elmer STA apparatus in air at the heating rate of 5 deg/min using 15 mg samples. The differential scanning calorimetry was carried out on a Perkin Elmer DSC8500 unit in an inert atmosphere at the heating rate of 5 deg/min. The thermomechanical curves were registered on a TA Instruments TMA Q400EM apparatus in air with the probe diameter of 3 mm, the loading force of 0.3 N, and the heating rate of 5 deg/min. Investigation of the strength characteristics of the glass-reinforced plastics was carried out according to GOST (State Standard) 4648-2014 on a Zwick/Roell Z020 versatile testing machine (Germany). The tests were carried out at 23 ± 2 °C with the speed of a moving crosshead of 1 mm/min. The support radius composed 2 ± 0.2 mm, the sample width was 15 ± 0.5 mm and its length was 20 times as much as the thicknesses. The samples from the nonextracted sheet of the glass-reinforced plastic were prepared by the mechanical processing according to GOST (State Standard) 26277.
Conclusions
A series of prepregs based on N,N'-hexamethylene bis(maleimide) and its compositions with diamines were prepared by dry application of the monomer mixtures on a fiberglass. It was shown that the addition of diamines allows one to obtain the flexible prepregs that do not embrittle upon different deformations and can be readily twisted to acquire different forms. The data of rheological and DSC studies were used to choose the optimal conditions for pressing of the glass-reinforced plastics. The temperature–time parameters of the resin curing, which characterize the conversion degree, were determined from the results of extraction and gel fractions of the polymers. The copolymerization of HMBMI with aniline fluorene or the siloxane-containing diamine ensured improvement of the bending strengths of the composites by 25–50% at the lower elasticity modulus, which testifies a reduction in the brittleness of the materials compared to the glass-reinforced plastics based on polyHMBMI. It was shown that the explored aromatic and siloxane-containing diamines only insignificantly reduce the thermal stability of the composites, while the temperature of 5% mass loss composes ~400 °C.
Acknowledgements
The authors are grateful to S. I. Kazakov, S. V. Kazakova, T. M. Pavlova, and O. E. Peksimov for spectral, thermal, rheological, and mechanical studies.
References
- S. V. Vinogradova, V. A. Vasnev, Ya. S. Vygodskii, Russ. Chem. Rev., 1996, 65, 249–277. DOI: 10.1070/RC1996v065n03ABEH000209
- R. J. Iredale, C. Ward, I. Hamerton, Prog. Polym. Sci., 2017, 69, 1–21. DOI: 10.1016/j.progpolymsci.2016.12.002
- E. S. Zrlenskii, A. M. Kuperman, Yu. A. Gorbatkina, V. G. Ivanova-Mumzhieva, A. A. Berlin, Ross. Khim. Zh., 2001, 45 (2), 56–74.
- D. B. Miracle, S. L. Donaldson, Bismaleimide Resins, in ASM Handbook, ASM Int., 2001, vol. 21, 1201.
- H. Stenzenberger, Br. Polym. J., 1988, 20, 383–396. DOI: 10.1002/pi.4980200503
- M. Frigione, J. M. Kenny, Adv. Polym. Technol., 2005, 24, 253–265. DOI: 10.1002/adv.20048
- M. Abbate, E. Martuscelli, P. Musto, G. Ragosta, J. Appl. Polym. Sci., 1997, 65, 979–990. DOI: 10.1002/(SICI)1097-4628(19970801)65:5<979::AID-APP16>3.0.CO;2-N
- X. Xiong, P. Chen, J. Zhang, Q. Yu, B. Wang, Thermochim. Acta, 2011, 514, 44–50. DOI: 10.1016/j.tca.2010.12.001
- H.-q. Yan, H.-q. Wang, J. Cheng, Eur. Polym. J., 2009, 45, 2383–2390. DOI: 10.1016/j.eurpolymj.2009.04.031
- G. Lubin, Handbook of Composites, Springer, 1982, 89–114. DOI: 10.1007/978-1-4615-7139-1
- S. P. Wilkinson, T. C. Ward, J. E. McGrath, Polymer, 1993, 34, 870–884. DOI: 10.1016/0032-3861(93)90376-L
- H. D. Stenzenberger, M. Herzog, P. Romer, Bismaleimide Resins: Past, Present, Future, Proc. 34th Int. SAMPE Symp., 1989, 1877.
- H. G. Recker, H. Tesch, T. Weber, V. Altstaedt, J. D. Boyd, US Patent 5532296, 1996.
- V. Gaina, C. Gaina, A. Stoleriu, D. Timpu, M. Sava, M. Rusu, Polym.-Plast. Technol. Eng., 1999, 38, 927–938. DOI: 10.1080/03602559909351622
- J. Kurdi, A. Kumar, J. Membr. Sci., 2006, 280, 234–244. DOI: 10.1016/j.memsci.2006.01.024
- I. Hamerton, P. Klewpatinond, Polym. Int., 2001, 50, 1309–1317. DOI: 10.1002/pi.776
- X. Liu, Y. Yu, S. Li, Eur. Polym. J., 2006, 42, 835–842. DOI: 10.1016/j.eurpolymj.2005.10.004
- J. Jin, J. Cui, X. Tang, Y. Ding, S. Li, J. Wang, Q. Zhao, X. Hua, X. Cai, Macromol. Chem. Phys., 1999, 200, 1956–1960. DOI: 10.1002/(SICI)1521-3935(19990801)200:8<1956::AID-MACP1956>3.0.CO;2-S
- H. D. Stenzenberger, P. König, High Perform. Polym., 1993, 5, 123–137. DOI: 10.1088/0954-0083/5/2/004
- S. Vinayagamoorthi, C. T. Vijayakumar, S. Alam, S. Nanjundan, Eur. Polym. J., 2009, 45, 1217–1231. DOI: 10.1016/j.eurpolymj.2008.12.026
- R. K. Jena, C. Y. Yue, M. M. Sk, K. Ghosh, RSC Adv., 2015, 5, 79888–79897. DOI: 10.1039/C5RA14474D
- M. Gaku, K. Suzuki, K. Nakamichi, US Patent 4110364, 1978.
- R.-H. Lin, W.-H. Lu, C.-W. Lin, Polymer, 2004, 45, 4423–4435. DOI: 10.1016/j.polymer.2004.04.050
- B. Kiskan, N. N. Ghosh, Y. Yagci, Polym. Int., 2011, 60, 167–177. DOI: 10.1002/pi.2961
- K. S. Santhosh Kumar, C. P. Reghunadhan Nair, R. Sadhana, K. N. Ninan, Eur. Polym. J., 2007, 43, 5084–5096. DOI: 10.1016/j.eurpolymj.2007.09.012
- T. Takeichi, Y. Saito, T. Agag, H. Muto, T. Kawauchi, Polymer, 2008, 49, 1173–1179. DOI: 10.1016/j.polymer.2008.01.041
- I. Hamerton, High Perform. Polym., 1996, 8, 83–95. DOI: 10.1088/0954-0083/8/1/006
- L. Jin, T. Agag, H. Ishida, Eur. Polym. J., 2010, 46, 354–363. DOI: 10.1016/j.eurpolymj.2009.09.013
- J. L. Hopewell, G. A. George, D. J .T. Hill, Polymer, 2000, 41, 8231–8239. DOI: 10.1016/S0032-3861(00)00193-2
- D. Kumar, G. M. Fohlen, J. A. Parker, J. Polym. Sci., Polym. Chem. Ed., 1983, 21, 245–267. DOI: 10.1002/pol.1983.170210125
- W. Wu, D. Wang, C. Ye, J. Appl. Polym. Sci., 1998, 70, 2471–2477. DOI: 10.1002/(SICI)1097-4628(19981219)70:12<2471::AID-APP20>3.0.CO;2-W
- S. V. Vinogradova, Ya. S. Vygodskii, V. A. Adigelazov, V. S. Papkov, I. I. Dubovik, G. L. Slonimskii, Ya. G. Urman, S. G. Alekseeva, Sh. T. Bagirov, V. V. Korshak, Polym. Sci. U.S.S.R., 1981, 23, 1935–1943.
- H. D. Stenzenberger, K. U. Heinen, D. O. Hummel, J. Polym. Sci., Polym. Chem. Ed., 1976, 14, 2911–2925. DOI: 10.1002/pol.1976.170141208
- N. Boens, F. C. De Schryver, G. Smets, J. Polym. Sci., Polym. Chem. Ed., 1975, 13, 201–213. DOI: 10.1002/pol.1975.170130119





